The anabolic response to protein ingestion during recovery from exercise has no upper limit in magnitude and duration in vivo in humans
- PMID: 38118410
- PMCID: PMC10772463
- DOI: 10.1016/j.xcrm.2023.101324
The anabolic response to protein ingestion during recovery from exercise has no upper limit in magnitude and duration in vivo in humans
Abstract
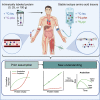
The belief that the anabolic response to feeding during postexercise recovery is transient and has an upper limit and that excess amino acids are being oxidized lacks scientific proof. Using a comprehensive quadruple isotope tracer feeding-infusion approach, we show that the ingestion of 100 g protein results in a greater and more prolonged (>12 h) anabolic response when compared to the ingestion of 25 g protein. We demonstrate a dose-response increase in dietary-protein-derived plasma amino acid availability and subsequent incorporation into muscle protein. Ingestion of a large bolus of protein further increases whole-body protein net balance, mixed-muscle, myofibrillar, muscle connective, and plasma protein synthesis rates. Protein ingestion has a negligible impact on whole-body protein breakdown rates or amino acid oxidation rates. These findings demonstrate that the magnitude and duration of the anabolic response to protein ingestion is not restricted and has previously been underestimated in vivo in humans.
Keywords: absorption; autophagy; bioavailability; de novo; digestion; intermittent fasting; mTOR; meal frequency; protein requirements; time-restricted feeding.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests G.A.A.v.L. is an employee of FrieslandCampina. The company had no role in funding, data collection and analysis, or preparation of the manuscript. See the following pages for a full overview of career funding for J.T. and L.J.C.v.L.: https://www.maastrichtuniversity.nl/j-trommelen and https://www.maastrichtuniversity.nl/ljc-van-loon.
Figures






References
-
- Phillips S.M., Tipton K.D., Aarsland A., Wolf S.E., Wolfe R.R. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am. J. Physiol. 1997;273:E99–E107. - PubMed
-
- Biolo G., Tipton K.D., Klein S., Wolfe R.R. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am. J. Physiol. 1997;273:E122–E129. - PubMed
-
- Trommelen J., Betz M.W., van Loon L.J.C. The Muscle Protein Synthetic Response to Meal Ingestion Following Resistance-Type Exercise. Sports Med. 2019;49:185–197. - PubMed
-
- Trommelen J., Holwerda A.M., Kouw I.W.K., Langer H., Halson S.L., Rollo I., Verdijk L.B., VAN Loon L.J.C. Resistance Exercise Augments Postprandial Overnight Muscle Protein Synthesis Rates. Med. Sci. Sports Exerc. 2016;48:2517–2525. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

