Potential therapeutic implications of histidine catabolism by the gut microbiota in NAFLD patients with morbid obesity
- PMID: 38118419
- PMCID: PMC10772641
- DOI: 10.1016/j.xcrm.2023.101341
Potential therapeutic implications of histidine catabolism by the gut microbiota in NAFLD patients with morbid obesity
Abstract
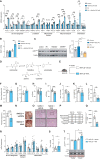
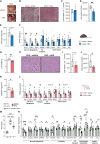
The gut microbiota contributes to the pathophysiology of non-alcoholic fatty liver disease (NAFLD). Histidine is a key energy source for the microbiota, scavenging it from the host. Its role in NAFLD is poorly known. Plasma metabolomics, liver transcriptomics, and fecal metagenomics were performed in three human cohorts coupled with hepatocyte, rodent, and Drosophila models. Machine learning analyses identified plasma histidine as being strongly inversely associated with steatosis and linked to a hepatic transcriptomic signature involved in insulin signaling, inflammation, and trace amine-associated receptor 1. Circulating histidine was inversely associated with Proteobacteria and positively with bacteria lacking the histidine utilization (Hut) system. Histidine supplementation improved NAFLD in different animal models (diet-induced NAFLD in mouse and flies, ob/ob mouse, and ovariectomized rats) and reduced de novo lipogenesis. Fecal microbiota transplantation (FMT) from low-histidine donors and mono-colonization of germ-free flies with Enterobacter cloacae increased triglyceride accumulation and reduced histidine content. The interplay among microbiota, histidine catabolism, and NAFLD opens therapeutic opportunities.
Keywords: Hut operon; NAFLD; Proteobacteria; amino acids; dysbiosis; hepatic disease; histidine; omics.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
-
- Quesada-Vázquez S., Colom-Pellicer M., Navarro-Masip È., Aragonès G., del Bas J.M., Caimari A., Escoté X. Supplementation with a Specific Combination of Metabolic Cofactors Ameliorates Non-Alcoholic Fatty Liver Disease and, Hepatic Fibrosis, and Insulin Resistance in Mice. Nutrients. 2021;13:3532. - PMC - PubMed
-
- Kasaoka S., Tsuboyama-Kasaoka N., Kawahara Y., Inoue S., Tsuji M., Ezaki O., Kato H., Tsuchiya T., Okuda H., Nakajima S. Histidine supplementation suppresses food intake and fat accumulation in rats. Nutrition. 2004;20:991–996. - PubMed
-
- Kennedy L., Hargrove L., Demieville J., Bailey J.M., Dar W., Polireddy K., Chen Q., Nevah Rubin M.I., Sybenga A., DeMorrow S., et al. Knockout of l-Histidine Decarboxylase Prevents Cholangiocyte Damage and Hepatic Fibrosis in Mice Subjected to High-Fat Diet Feeding via Disrupted Histamine/Leptin Signaling. Am. J. Pathol. 2018;188:600–615. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

