Unified framework for patient-derived, tumor-organoid-based predictive testing of standard-of-care therapies in metastatic colorectal cancer
- PMID: 38118423
- PMCID: PMC10783557
- DOI: 10.1016/j.xcrm.2023.101335
Unified framework for patient-derived, tumor-organoid-based predictive testing of standard-of-care therapies in metastatic colorectal cancer
Abstract
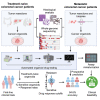
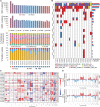
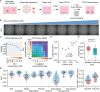
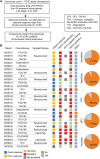
Predictive drug testing of patient-derived tumor organoids (PDTOs) holds promise for personalizing treatment of metastatic colorectal cancer (mCRC), but prospective data are limited to chemotherapy regimens with conflicting results. We describe a unified framework for PDTO-based predictive testing across standard-of-care chemotherapy and biologic and targeted therapy options. In an Australian community cohort, PDTO predictions based on treatment-naive patients (n = 56) and response rates from first-line mCRC clinical trials achieve 83% accuracy for forecasting responses in patients receiving palliative treatments (18 patients, 29 treatments). Similar assay accuracy is achieved in a prospective study of third-line or later mCRC treatment, AGITG FORECAST-1 (n = 30 patients). "Resistant" predictions are associated with inferior progression-free survival; misclassification rates are similar by regimen. Liver metastases are the optimal site for sampling, with testing achievable within 7 weeks for 68.8% cases. Our findings indicate that PDTO drug panel testing can provide predictive information for multifarious standard-of-care therapies for mCRC.
Keywords: colorectal cancer; patient-derived tumor organoid; precision medicine; predictive drug testing.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021;71:209–249. - PubMed
-
- Van Cutsem E., Köhne C.H., Láng I., Folprecht G., Nowacki M.P., Cascinu S., Shchepotin I., Maurel J., Cunningham D., Tejpar S., et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J. Clin. Oncol. 2011;29:2011–2019. - PubMed
-
- Tejpar S., Stintzing S., Ciardiello F., Tabernero J., Van Cutsem E., Beier F., Esser R., Lenz H.J., Heinemann V. Prognostic and Predictive Relevance of Primary Tumor Location in Patients With RAS Wild-Type Metastatic Colorectal Cancer: Retrospective Analyses of the CRYSTAL and FIRE-3 Trials. JAMA Oncol. 2017;3:194–201. - PMC - PubMed
-
- Douillard J.Y., Siena S., Cassidy J., Tabernero J., Burkes R., Barugel M., Humblet Y., Bodoky G., Cunningham D., Jassem J., et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann. Oncol. 2014;25:1346–1355. - PubMed
-
- Peeters M., Oliner K.S., Price T.J., Cervantes A., Sobrero A.F., Ducreux M., Hotko Y., André T., Chan E., Lordick F., et al. Analysis of KRAS/NRAS Mutations in a Phase III Study of Panitumumab with FOLFIRI Compared with FOLFIRI Alone as Second-line Treatment for Metastatic Colorectal Cancer. Clin. Cancer Res. 2015;21:5469–5479. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

