Use of Quantitative CT Imaging to Identify Bronchial Thermoplasty Responders
- PMID: 38123124
- PMCID: PMC11026166
- DOI: 10.1016/j.chest.2023.12.015
Use of Quantitative CT Imaging to Identify Bronchial Thermoplasty Responders
Abstract
Background: Bronchial thermoplasty (BT) is a treatment for patients with poorly controlled, severe asthma. However, predictors of treatment response to BT are defined poorly.
Research question: Do baseline radiographic and clinical characteristics exist that predict response to BT?
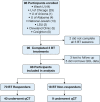
Study design and methods: We conducted a longitudinal prospective cohort study of participants with severe asthma receiving BT across eight academic medical centers. Participants received three separate BT treatments and were monitored at 3-month intervals for 1 year after BT. Similar to prior studies, a positive response to BT was defined as either improvement in Asthma Control Test results of ≥ 3 or Asthma Quality of Life Questionnaire of ≥ 0.5. Regression analyses were used to evaluate the association between pretreatment clinical and quantitative CT scan measures with subsequent BT response.
Results: From 2006 through 2017, 88 participants received BT, with 70 participants (79.5%) identified as responders by Asthma Control Test or Asthma Quality of Life Questionnaire criteria. Responders were less likely to undergo an asthma-related ICU admission in the prior year (3% vs 25%; P = .01). On baseline quantitative CT imaging, BT responders showed less air trapping percentage (OR, 0.90; 95% CI, 0.82-0.99; P = .03), a greater Jacobian determinant (OR, 1.49; 95% CI, 1.05-2.11), greater SD of the Jacobian determinant (OR, 1.84; 95% CI, 1.04-3.26), and greater anisotropic deformation index (OR, 3.06; 95% CI, 1.06-8.86).
Interpretation: To our knowledge, this is the largest study to evaluate baseline quantitative CT imaging and clinical characteristics associated with BT response. Our results show that preservation of normal lung expansion, indicated by less air trapping, a greater magnitude of isotropic expansion, and greater within-lung spatial variation on quantitative CT imaging, were predictors of future BT response.
Trial registry: ClinicalTrials.gov; No.: NCT01185275; URL: www.
Clinicaltrials: gov.
Keywords: air trapping; bronchial thermoplasty; lung deformation; quantitative CT; severe asthma.
Copyright © 2023 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Financial/Nonfinancial Disclosures The authors have reported to CHEST the following: J. G. K. reports personal fees and nonfinancial support from Genentech and Sanofi. C. S. H. has received speaking fees from VIDA, Boehringer Ingelheim, Polarean. A. S. has received consulting fees from Entana Pharmaceuticals. S. P. is an employee and stock option holder of VIDA. D. K. H. is a consultant for Boston Scientific. J. T. is on the Astra Zeneca Study steering committee. M. E. W. has received consulting, advisory, or speaking honoraria from Amgen, AstraZeneca, Avalo Therapeutics, Boehringer Ingelheim, Cerecor, Cohero Health, Cytoreason, Eli Lilly, Equillium, Glaxosmithkline, Incyte, Kinaset, Novartis, Om Pharma, Overtone Therapeutics/Foresite Labs, Phylaxis, Pulmatrix, Rapt Therapeutics, Regeneron, Restorbio, Roche/Genentech, Sanofi/Genzyme, Sentien, Sound Biologics, Tetherex Pharmaceuticals, Teva, and Upstream Bio. M. C. reports institutional grant funding from the National Institutes of Health, American Lung Association, Patient Centered Outcomes Research Institute, AstraZeneca, GSK, Novartis, Pulmatrix, Sanofi-Aventis, and Shionogi; consulting fees from Genentech, Teva, Sanofi-Aventis, Merck, Novartis, Arrowhead OM Pharma, and Allakos; payment for speaker’s bureau activities for Amgen, AstraZeneca, Genentech, GSK, Regeneron, Sanofi-Aventis, and Teva; and royalties from Elsevier. None declared (M. S., D. L., C. W. G., T. K., M. C. M., J. B., K. B. S., S. E., Z. D. L. E. M., R. T., A. S., X. S., J. C.).
Figures

Comment in
-
Quantitative Imaging and Bronchial Thermoplasty: Technically Impressive But Clinically Uncertain.Chest. 2024 Apr;165(4):755-756. doi: 10.1016/j.chest.2024.02.024. Chest. 2024. PMID: 38599744 No abstract available.
References
-
- Nurmagambetov T., Kuwahara R., Garbe P. The economic burden of asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15(3):348–356. - PubMed
-
- Chung K.F., Wenzel S.E., Brozek J.L., et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. - PubMed
-
- Pretolani M., Dombret M.C., Thabut G., et al. Reduction of airway smooth muscle mass by bronchial thermoplasty in patients with severe asthma. Am J Respir Crit Care Med. 2014;190(12):1452–1454. - PubMed

