Comparison of Tricaine Methanesulfonate (MS-222) and Alfaxalone Anesthesia in Zebrafish (Danio rerio)
- PMID: 38123147
- PMCID: PMC10844738
- DOI: 10.30802/AALAS-JAALAS-23-000058
Comparison of Tricaine Methanesulfonate (MS-222) and Alfaxalone Anesthesia in Zebrafish (Danio rerio)
Abstract
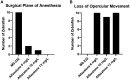
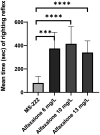
The research use of zebrafish has risen exponentially over the past decade while anesthetic options have remained largely unchanged.6 ricaine methanesulfonate (MS-222) is widely accepted as an anesthetic for routine husbandry procedures, however it has limitations and safety concerns. 11 A greater variety of effective anesthetic options for surgical procedures would be advantageous for the research community. Adult zebrafish were randomly assigned to one of the following groups (n = 10, 5 males and 5 females): 200 mg/L MS-222; 6-, 10-, 13-, and 16-mg/L alfaxalone, and control. All zebrafish in the MS-222 group reached a surgical plane of anesthesia within 95 ± 32 s. By contrast, only 2 of 10, 1 of 10, 0 of 10, and 0 of 4 of the 6, 10, 13, and 16 mg/L alfaxalone groups, respectively, reached a surgical plane of anesthesia within the allotted 10-min period. Recovery time was also significantly slower in the alfaxalone groups as compared with MS-222, with some fish taking greater than 10 min to recover. In addition, 33 of 34 zebrafish (the 16 mg/L group was not completed due to safety concerns) in the alfaxalone groups lost opercular movements for greater than one minute during their anesthetic event and had to be removed to the recovery tank. The results demonstrated that alfaxalone was unable to provide a reliable and safe surgical plane of anesthesia at any of the drug doses tested. Therefore, we recommend alfaxalone not be used as an anesthetic for painful procedures on zebrafish and conclude that MS-222 remains a more viable anesthetic for immersion anesthesia in zebrafish.
Figures



References
-
- Collymore C. Anesthesia, analgesia, and euthanasia of the laboratory zebrafish, p 403-413. In: Cartner SC. editor. The Zebrafish in Biomedical Research. San Diego (CA): Elsevier.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

