Engineering a synthetic energy-efficient formaldehyde assimilation cycle in Escherichia coli
- PMID: 38123535
- PMCID: PMC10733421
- DOI: 10.1038/s41467-023-44247-2
Engineering a synthetic energy-efficient formaldehyde assimilation cycle in Escherichia coli
Abstract
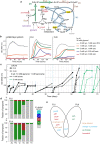
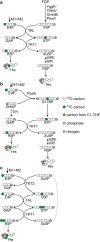
One-carbon (C1) substrates, such as methanol or formate, are attractive feedstocks for circular bioeconomy. These substrates are typically converted into formaldehyde, serving as the entry point into metabolism. Here, we design an erythrulose monophosphate (EuMP) cycle for formaldehyde assimilation, leveraging a promiscuous dihydroxyacetone phosphate dependent aldolase as key enzyme. In silico modeling reveals that the cycle is highly energy-efficient, holding the potential for high bioproduct yields. Dissecting the EuMP into four modules, we use a stepwise strategy to demonstrate in vivo feasibility of the modules in E. coli sensor strains with sarcosine as formaldehyde source. From adaptive laboratory evolution for module integration, we identify key mutations enabling the accommodation of the EuMP reactions with endogenous metabolism. Overall, our study demonstrates the proof-of-concept for a highly efficient, new-to-nature formaldehyde assimilation pathway, opening a way for the development of a methylotrophic platform for a C1-fueled bioeconomy in the future.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

