Inflammasome activity is controlled by ZBTB16-dependent SUMOylation of ASC
- PMID: 38123560
- PMCID: PMC10733316
- DOI: 10.1038/s41467-023-43945-1
Inflammasome activity is controlled by ZBTB16-dependent SUMOylation of ASC
Abstract
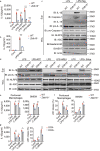
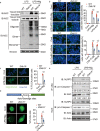
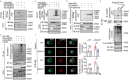
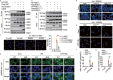
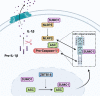
Inflammasome activity is important for the immune response and is instrumental in numerous clinical conditions. Here we identify a mechanism that modulates the central Caspase-1 and NLR (Nod-like receptor) adaptor protein ASC (apoptosis-associated speck-like protein containing a CARD). We show that the function of ASC in assembling the inflammasome is controlled by its modification with SUMO (small ubiquitin-like modifier) and identify that the nuclear ZBTB16 (zinc-finger and BTB domain-containing protein 16) promotes this SUMOylation. The physiological significance of this activity is demonstrated through the reduction of acute inflammatory pathogenesis caused by a constitutive hyperactive inflammasome by ablating ZBTB16 in a mouse model of Muckle-Wells syndrome. Together our findings identify an further mechanism by which ZBTB16-dependent control of ASC SUMOylation assembles the inflammasome to promote this pro-inflammatory response.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

