Targeting hypoxia-inducible factors: therapeutic opportunities and challenges
- PMID: 38123660
- PMCID: PMC12337356
- DOI: 10.1038/s41573-023-00848-6
Targeting hypoxia-inducible factors: therapeutic opportunities and challenges
Abstract
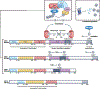
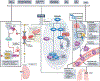
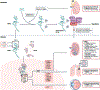
Hypoxia-inducible factors (HIFs) are highly conserved transcription factors that are crucial for adaptation of metazoans to limited oxygen availability. Recently, HIF activation and inhibition have emerged as therapeutic targets in various human diseases. Pharmacologically desirable effects of HIF activation include erythropoiesis stimulation, cellular metabolism optimization during hypoxia and adaptive responses during ischaemia and inflammation. By contrast, HIF inhibition has been explored as a therapy for various cancers, retinal neovascularization and pulmonary hypertension. This Review discusses the biochemical mechanisms that control HIF stabilization and the molecular strategies that can be exploited pharmacologically to activate or inhibit HIFs. In addition, we examine medical conditions that benefit from targeting HIFs, the potential side effects of HIF activation or inhibition and future challenges in this field.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests
H.K.E., B.B. and X.Y. received research funding through a contract between Akebia Therapeutics and UTHealth to support a clinical trial on the effect of vadadustat in hospitalized patients with COVID-19 (
Additional information
Figures

References
-
- Taylor CT & McElwain JC Ancient atmospheres and the evolution of oxygen sensing via the hypoxia-inducible factor in metazoans. Physiology 25, 272–279 (2010). - PubMed
-
- Semenza GL, Roth PH, Fang HM & Wang GL Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 269, 23757–23763 (1994). - PubMed
-
- Liu Y, Cox SR, Morita T & Kourembanas S Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5′ enhancer. Circ. Res. 77, 638–643 (1995). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

