Radium-223 in women with hormone receptor-positive bone-metastatic breast cancer receiving endocrine therapy: pooled analysis of two international, phase 2, randomized, double-blind, placebo-controlled trials
- PMID: 38123789
- PMCID: PMC10948526
- DOI: 10.1007/s10549-023-07147-z
Radium-223 in women with hormone receptor-positive bone-metastatic breast cancer receiving endocrine therapy: pooled analysis of two international, phase 2, randomized, double-blind, placebo-controlled trials
Abstract
Background: Most women with advanced breast cancer have skeletal metastases. Radium-223 is an alpha-emitting radionuclide that selectively targets areas of bone metastases.
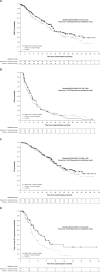
Methods: Two double-blind, placebo-controlled studies of radium-223 were conducted in women with hormone receptor-positive (HR+), bone-predominant metastatic breast cancer. All patients received endocrine therapy (ET), as a single agent of the investigator's choice (Study A) or exemestane + everolimus (Study B). Patients were randomized to receive radium-223 (55 kBq/kg) or placebo intravenously every 4 weeks for six doses. Accrual was halted following unblinded interim analyses per protocol amendments, and both studies were terminated. We report pooled analyses of symptomatic skeletal event-free survival (SSE-FS; primary endpoint), radiologic progression-free survival (rPFS) and overall survival (OS; secondary), and time to bone alkaline phosphatase (ALP) progression (exploratory).
Results: In total, 382 patients were enrolled, and 196 SSE-FS events (70% planned total) were recorded. Hazard ratios (95% confidence intervals) and nominal p values for radium-223 + ET versus placebo + ET were: SSE-FS 0.809 (0.610-1.072), p = 0.1389; rPFS 0.956 (0.759-1.205), p = 0.7039; OS 0.889 (0.660-1.199), p = 0.4410; and time to bone ALP progression 0.593 (0.379-0.926), p = 0.0195. Radium-223- or placebo-related treatment-emergent adverse events were reported in 50.3% versus 35.1% of patients (grade 3/4: 25.7% vs. 8.5%), with fractures/bone-associated events in 23.5% versus 23.9%.
Conclusions: In patients with HR+ bone-metastatic breast cancer, numeric differences favoring radium-223 + ET over placebo + ET for the primary SSE-FS endpoint were suggestive of efficacy, in line with the primary outcome measure used in the underlying phase 2 studies. No similar evidence of efficacy was observed for secondary progression or survival endpoints. Adverse events were more frequent with radium-223 + ET versus placebo + ET, but the safety profile of the combination was consistent with the safety profiles of the component drugs. Clinical trial registration numbers Study A: NCT02258464, registered October 7, 2014. Study B: NCT02258451, registered October 7, 2014.
Keywords: Bone metastasis; Breast cancer; Endocrine therapy; Hormone receptor positive; Radium-223; Symptomatic skeletal event-free survival.
© 2023. The Author(s).
Conflict of interest statement
HSR: Grants to institution from Astellas, AstraZeneca, Ayala, Boehringer Ingelheim, Daiichi, Gilead, Lilly, Macrogenics, Merck, Novartis, Pfizer, Polyphor, Roche, Seattle Genetics, and Sermonix; honoraria from Mylan, NAPO, Puma, and Samsung. CVP: Research grant from Bayer for a study outside of the one associated with this manuscript; royalties from UpToDate; board member (unpaid) of Cancer and Bone Society, an independent organization that promotes international collaborations within the cancer and bone field in both clinical and basic research. PN: Research funding from Kom op Tegen Kanker; consulting fees from AstraZeneca, Lilly, Novartis, Pfizer, and Roche; consulting or advisory roles for Lilly, Novartis, Pfizer, and Roche. ID, YKC, EVA, BN: No conflicts to disclose. SCL: Research grants from ACT Genomics, Eisai, Pfizer, and Taiho; consulting/advisory roles for, and payment/honoraria from, AstraZeneca, Daiichi-Sankyo, Lilly, Merck Sharp & Dohme, Novartis, Pfizer, and Roche; travel/meeting attendance support from Pfizer and Roche. MC: Payment and/or honoraria from Amgen, Gilead, GSK, Merck Sharp & Dohme, Novartis, Pfizer, and Seagen (to institution) and from Lilly, Pierre Fabre, and Sanofi (to self). EB: Consulting fees from Daiichi-Sankyo, Pfizer, and Sandoz; payment and/or honoraria from Eli Lilly, Pfizer, and Seagen; travel/meeting attendance support from Pfizer and Sandoz; consultancy/advisory role for Daiichi-Sankyo. JMS: Employee of Bayer; stock ownership in Bayer AG; leadership and/or committee roles in Pharmaceutical Industry Working Group on Estimands in Oncology, Society for Clinical Biostatistics Subcommittee for Statistics in Regulatory Affairs, and American Statistical Association. RL, DU, VW: Employee of Bayer. REC: Consulting fees from Sanofi; payment/honoraria from Amgen and Beigene; patent for MAF gene test and stock options with Inbiomotion; consultancy/advisory role for AstraZeneca.
Figures
References
-
- Coleman R, Brown J, Rathbone E, Flanagan L, Reid A, Kendall J, Howell S, Twelves C, Palmieri C, Anand A, MacPherson I, Brown S. CApecitabine plus radium-223 (Xofigo) in breast cancer patients with BONe metastases (CARBON): study protocol for a phase IB/IIA randomised controlled trial. Trials. 2020;21:89. doi: 10.1186/s13063-019-3643-6. - DOI - PMC - PubMed
-
- Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fossa SD, Chodacki A, Wiechno P, Logue J, Seke M, Widmark A, Johannessen DC, Hoskin P, Bottomley D, James ND, Solberg A, Syndikus I, Kliment J, Wedel S, Boehmer S, Dall'Oglio M, Franzen L, Coleman R, Vogelzang NJ, O'Bryan-Tear CG, Staudacher K, Garcia-Vargas J, Shan M, Bruland OS, Sartor O, Investigators ALSYMPCA. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

