Chemoselective umpolung of thiols to episulfoniums for cysteine bioconjugation
- PMID: 38123842
- PMCID: PMC10914617
- DOI: 10.1038/s41557-023-01388-7
Chemoselective umpolung of thiols to episulfoniums for cysteine bioconjugation
Erratum in
-
Author Correction: Chemoselective umpolung of thiols to episulfoniums for cysteine bioconjugation.Nat Chem. 2024 Mar;16(3):476. doi: 10.1038/s41557-024-01475-3. Nat Chem. 2024. PMID: 38360934 Free PMC article. No abstract available.
Abstract
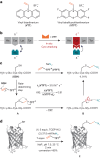
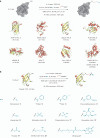
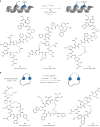
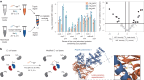
Cysteine conjugation is an important tool in protein research and relies on fast, mild and chemoselective reactions. Cysteinyl thiols can either be modified with prefunctionalized electrophiles, or converted into electrophiles themselves for functionalization with selected nucleophiles in an independent step. Here we report a bioconjugation strategy that uses a vinyl thianthrenium salt to transform cysteine into a highly reactive electrophilic episulfonium intermediate in situ, to enable conjugation with a diverse set of bioorthogonal nucleophiles in a single step. The reactivity profile can connect several nucleophiles to biomolecules through a short and stable ethylene linker, ideal for introduction of infrared labels, post-translational modifications or NMR probes. In the absence of reactive exogenous nucleophiles, nucleophilic amino acids can react with the episulfonium intermediate for native peptide stapling and protein-protein ligation. Ready synthetic access to isotopologues of vinyl thianthrenium salts enables applications in quantitative proteomics. Such diverse applications demonstrate the utility of vinyl-thianthrenium-based bioconjugation as a fast, selective and broadly applicable tool for chemical biology.
© 2023. The Author(s).
Conflict of interest statement
F.J. and T.R. may benefit from potential royalty income related to vinyl-thianthrenium-based reagents. The other authors declare no competing interests.
Figures

References
-
- Fodje MN, Al-Karadaghi S. Occurrence, conformational features and amino acid propensities for the π-helix. Protein Eng. Des. Sel. 2002;15:353–358. - PubMed
-
- Gunnoo SB, Madder A. Chemical protein modification through cysteine. ChemBioChem. 2016;17:529–553. - PubMed
-
- Seki Y, et al. Transition metal-free tryptophan-selective bioconjugation of proteins. J. Am. Chem. Soc. 2016;138:10798–10801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

