Genetic ablation of Sarm1 attenuates expression and mislocalization of phosphorylated TDP-43 after mouse repetitive traumatic brain injury
- PMID: 38124145
- PMCID: PMC10731794
- DOI: 10.1186/s40478-023-01709-4
Genetic ablation of Sarm1 attenuates expression and mislocalization of phosphorylated TDP-43 after mouse repetitive traumatic brain injury
Abstract
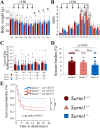
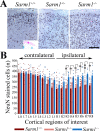
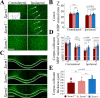
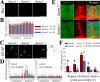
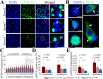
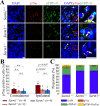
Traumatic brain injury (TBI), particularly when moderate-to-severe and repetitive, is a strong environmental risk factor for several progressive neurodegenerative disorders. Mislocalization and deposition of transactive response DNA binding protein 43 (TDP-43) has been reported in both TBI and TBI-associated neurodegenerative diseases. It has been hypothesized that axonal pathology, an early event after TBI, may promote TDP-43 dysregulation and serve as a trigger for neurodegenerative processes. We sought to determine whether blocking the prodegenerative Sarm1 (sterile alpha and TIR motif containing 1) axon death pathway attenuates TDP-43 pathology after TBI. We subjected 111 male Sarm1 wild type, hemizygous, and knockout mice to moderate-to-severe repetitive TBI (rTBI) using a previously established injury paradigm. We conducted serial neurological assessments followed by histological analyses (NeuN, MBP, Iba-1, GFAP, pTDP-43, and AT8) at 1 month after rTBI. Genetic ablation of the Sarm1 gene attenuated the expression and mislocalization of phosphorylated TDP-43 (pTDP-43) and accumulation of pTau. In addition, Sarm1 knockout mice had significantly improved cortical neuronal and axonal integrity, functional deficits, and improved overall survival after rTBI. In contrast, removal of one Sarm1 allele delayed, but did not prevent, neurological deficits and neuroaxonal loss. Nevertheless, Sarm1 haploinsufficient mice showed significantly less microgliosis, pTDP-43 pathology, and pTau accumulation when compared to wild type mice. These data indicate that the Sarm1-mediated prodegenerative pathway contributes to pathogenesis in rTBI including the pathological accumulation of pTDP-43. This suggests that anti-Sarm1 therapeutics are a viable approach for preserving neurological function after moderate-to-severe rTBI.
Keywords: Axon; Behavior; Brain injury; Glial scar; Haploinsufficiency; Interleukin; Neurodegeneration; SARM1; TDP-43; Tau.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Genetic Ablation of Sarm1 Mitigates Disease Acceleration after Traumatic Brain Injury in the SOD1G93A Transgenic Mouse Model of Amyotrophic Lateral Sclerosis.Ann Neurol. 2025 May;97(5):963-975. doi: 10.1002/ana.27174. Epub 2025 Jan 10. Ann Neurol. 2025. PMID: 39791335
-
Repeated mild traumatic brain injury triggers pathology in asymptomatic C9ORF72 transgenic mice.Brain. 2023 Dec 1;146(12):5139-5152. doi: 10.1093/brain/awad264. Brain. 2023. PMID: 37527465 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Transactive Response DNA-Binding Protein 43 Abnormalities after Traumatic Brain Injury.J Neurotrauma. 2019 Jan 1;36(1):87-99. doi: 10.1089/neu.2017.5491. Epub 2018 Aug 10. J Neurotrauma. 2019. PMID: 29901412
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
Cited by
-
Genetic Ablation of Sarm1 Mitigates Disease Acceleration after Traumatic Brain Injury in the SOD1G93A Transgenic Mouse Model of Amyotrophic Lateral Sclerosis.Ann Neurol. 2025 May;97(5):963-975. doi: 10.1002/ana.27174. Epub 2025 Jan 10. Ann Neurol. 2025. PMID: 39791335
References
-
- Alexandris AS, Lee Y, Lehar M, Alam Z, Samineni P, Tripathi SJ, et al. Traumatic axonopathy in spinal tracts after impact acceleration head injury: ultrastructural observations and evidence of SARM1-dependent axonal degeneration. Exp Neurol. 2023;359:114252. doi: 10.1016/j.expneurol.2022.114252. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

