Electrical stimulation affects the differentiation of transplanted regionally specific human spinal neural progenitor cells (sNPCs) after chronic spinal cord injury
- PMID: 38124191
- PMCID: PMC10734202
- DOI: 10.1186/s13287-023-03597-w
Electrical stimulation affects the differentiation of transplanted regionally specific human spinal neural progenitor cells (sNPCs) after chronic spinal cord injury
Abstract
Background: There are currently no effective clinical therapies to ameliorate the loss of function that occurs after spinal cord injury. Electrical stimulation of the rat spinal cord through the rat tail has previously been described by our laboratory. We propose combinatorial treatment with human induced pluripotent stem cell-derived spinal neural progenitor cells (sNPCs) along with tail nerve electrical stimulation (TANES). The purpose of this study was to examine the influence of TANES on the differentiation of sNPCs with the hypothesis that the addition of TANES would affect incorporation of sNPCs into the injured spinal cord, which is our ultimate goal.
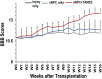
Methods: Chronically injured athymic nude rats were allocated to one of three treatment groups: injury only, sNPC only, or sNPC + TANES. Rats were sacrificed at 16 weeks post-transplantation, and tissue was processed and analyzed utilizing standard histological and tissue clearing techniques. Functional testing was performed. All quantitative data were presented as mean ± standard error of the mean. Statistics were conducted using GraphPad Prism.
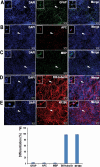
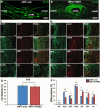
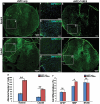
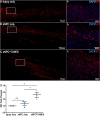
Results: We found that sNPCs were multi-potent and retained the ability to differentiate into mainly neurons or oligodendrocytes after this transplantation paradigm. The addition of TANES resulted in more transplanted cells differentiating into oligodendrocytes compared with no TANES treatment, and more myelin was found. TANES not only promoted significantly higher numbers of sNPCs migrating away from the site of injection but also influenced long-distance axonal/dendritic projections especially in the rostral direction. Further, we observed localization of synaptophysin on SC121-positive cells, suggesting integration with host or surrounding neurons, and this finding was enhanced when TANES was applied. Also, rats that were transplanted with sNPCs in combination with TANES resulted in an increase in serotonergic fibers in the lumbar region. This suggests that TANES contributes to integration of sNPCs, as well as activity-dependent oligodendrocyte and myelin remodeling of the chronically injured spinal cord.
Conclusions: Together, the data suggest that the added electrical stimulation promoted cellular integration and influenced the fate of human induced pluripotent stem cell-derived sNPCs transplanted into the injured spinal cord.
Keywords: Cell therapy; Human induced pluripotent stem cells; Spinal cord injury; Spinal neuronal progenitor cells; Tail nerve electrical stimulation.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Zhang S, Huang F, Gates M, White J, Holmberg EG. Tail nerve electrical stimulation induces body weight-supported stepping in rats with spinal cord injury. J Neurosci Methods. 2010;187(2):183–9. - PubMed
-
- Darrow D, Balser D, Netoff T, Krassioukov A, Phillips A, Parr A, Samadani U. Epidural spinal cord stimulation facilitates immediate restoration of dormant motor and autonomic supraspinal pathways after chronic neurologically complete spinal Cord Injury. J Neurotrauma. 2019;36(15):2325–36. - PMC - PubMed
-
- Kim TH, Kim S, Lee S, Lee JP, Snyder EY, Kook IP. Human neurospheres derived from the fetal central nervous system are regionally and temporally specified but are not committed. Exp Neurol. 2006;199(1):222–35. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

