Extracellular vesicle-associated cholesterol supports the regenerative functions of macrophages in the brain
- PMID: 38124258
- PMCID: PMC10733568
- DOI: 10.1002/jev2.12394
Extracellular vesicle-associated cholesterol supports the regenerative functions of macrophages in the brain
Abstract
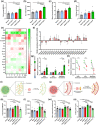
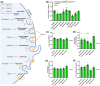
Macrophages play major roles in the pathophysiology of various neurological disorders, being involved in seemingly opposing processes such as lesion progression and resolution. Yet, the molecular mechanisms that drive their harmful and benign effector functions remain poorly understood. Here, we demonstrate that extracellular vesicles (EVs) secreted by repair-associated macrophages (RAMs) enhance remyelination ex vivo and in vivo by promoting the differentiation of oligodendrocyte precursor cells (OPCs). Guided by lipidomic analysis and applying cholesterol depletion and enrichment strategies, we find that EVs released by RAMs show markedly elevated cholesterol levels and that cholesterol abundance controls their reparative impact on OPC maturation and remyelination. Mechanistically, EV-associated cholesterol was found to promote OPC differentiation predominantly through direct membrane fusion. Collectively, our findings highlight that EVs are essential for cholesterol trafficking in the brain and that changes in cholesterol abundance support the reparative impact of EVs released by macrophages in the brain, potentially having broad implications for therapeutic strategies aimed at promoting repair in neurodegenerative disorders.
Keywords: cholesterol; extracellular vesicle; oligodendrocyte precursor cell differentiation; remyelination; repair-associated macrophage.
© 2023 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Ahsan, F. , Rivas, I. P. , Khan, M. A. , & Torres Suarez, A. I. (2002). Targeting to macrophages: Role of physicochemical properties of particulate carriers–liposomes and microspheres–on the phagocytosis by macrophages. Journal of Controlled Release, 79, 29–40. - PubMed
-
- Ajami, B. , Bennett, J. L. , Krieger, C. , McNagny, K. M. , & Rossi, F. M. (2011). Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nature Neuroscience, 14, 1142–1149. - PubMed
-
- Aydınlı, F. , Er, S. , & Kerman, B. E. (2022). Two phases of macrophages: Inducing maturation and death of oligodendrocytes in vitro co‐culture. Journal of Neuroscience Methods, 382, 109723. - PubMed
-
- Berghoff, S. A. , Gerndt, N. , Winchenbach, J. , Stumpf, S. K. , Hosang, L. , Odoardi, F. , Ruhwedel, T. , Bohler, C. , Barrette, B. , Stassart, R. , Liebetanz, D. , Dibaj, P. , Möbius, W. , Edgar, J. M. , & Saher, G. (2017). Dietary cholesterol promotes repair of demyelinated lesions in the adult brain. Nature Communications, 8, 14241. - PMC - PubMed
-
- Berghoff, S. A. , Spieth, L. , & Saher, G. (2022). Local cholesterol metabolism orchestrates remyelination. Trends in Neuroscience (Tins), 45, 272–283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

