Bone morphogenetic protein signaling: the pathway and its regulation
- PMID: 38124338
- PMCID: PMC10847725
- DOI: 10.1093/genetics/iyad200
Bone morphogenetic protein signaling: the pathway and its regulation
Abstract
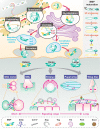
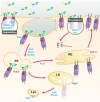
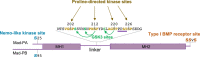
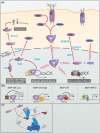
In the mid-1960s, bone morphogenetic proteins (BMPs) were first identified in the extracts of bone to have the remarkable ability to induce heterotopic bone. When the Drosophila gene decapentaplegic (dpp) was first identified to share sequence similarity with mammalian BMP2/BMP4 in the late-1980s, it became clear that secreted BMP ligands can mediate processes other than bone formation. Following this discovery, collaborative efforts between Drosophila geneticists and mammalian biochemists made use of the strengths of their respective model systems to identify BMP signaling components and delineate the pathway. The ability to conduct genetic modifier screens in Drosophila with relative ease was critical in identifying the intracellular signal transducers for BMP signaling and the related transforming growth factor-beta/activin signaling pathway. Such screens also revealed a host of genes that encode other core signaling components and regulators of the pathway. In this review, we provide a historical account of this exciting time of gene discovery and discuss how the field has advanced over the past 30 years. We have learned that while the core BMP pathway is quite simple, composed of 3 components (ligand, receptor, and signal transducer), behind the versatility of this pathway lies multiple layers of regulation that ensures precise tissue-specific signaling output. We provide a sampling of these discoveries and highlight many questions that remain to be answered to fully understand the complexity of BMP signaling.
Keywords: BMP signaling; DV patterning; Dpp; FlyBook; Gbb; NMJ; Sax; Tkv; morphogen gradient; wing patterning.
© The Author(s) 2023. Published by Oxford University Press on behalf of The Genetics Society of America.
Conflict of interest statement
Conflicts of interest The author(s) declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

