Invasive pneumococcal disease surveillance in Canada, 2020
- PMID: 38124782
- PMCID: PMC10732480
Invasive pneumococcal disease surveillance in Canada, 2020
Abstract
Background: Invasive pneumococcal disease (IPD), which is caused by Streptococcus pneumoniae, has been a nationally notifiable disease in Canada since 2000. The use of conjugate vaccines has markedly decreased the incidence of IPD in Canada; however, the distribution of serotypes has shifted in favour of non-vaccine types. This report summarizes the demographics, serotypes and antimicrobial resistance of IPD infections in Canada in 2020.
Methods: The Public Health Agency of Canada's National Microbiology Laboratory (Winnipeg, Manitoba) collaborates with provincial and territorial public health laboratories to conduct national surveillance of IPD. A total of 2,108 IPD isolates were reported in 2020. Serotyping was performed by Quellung reaction and antimicrobial susceptibilities were determined in collaboration with the University of Manitoba/Canadian Antimicrobial Resistance Alliance. Population-based IPD incidence rates were obtained through the Canadian Notifiable Disease Surveillance System.
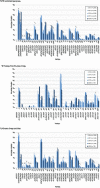
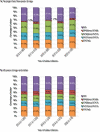
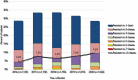
Results: Overall incidence of IPD in Canada decreased significantly from 11.5 (95% confidence interval [CI]: 10.1-13.1) to 6.0 (95% CI: 5.0-7.2), and from 10.0 (95% CI: 9.7-10.3) to 5.9 (95% CI: 5.7-6.2) cases per 100,000 from 2019 to 2020; in those younger than five years and those five years and older, respectively. The most common serotypes overall were 4 (11.2%, n=237), 3 (10.9%, n=229) and 8 (7.2%, n=151). From 2016 to 2020, serotypes with increasing trends (p<0.05) included 4 (6.4%-11.2%), 3 (9.5%-10.9%), 8 (5.2%-7.2%) and 12F (3.6%-5.7%). The overall prevalence of PCV13 serotypes increased over the same period (30.3%-34.9%, p<0.05). Antimicrobial resistance rates in 2020 included 23.0% clarithromycin and 9.9% penicillin (IV meningitis breakpoints). Multidrug-resistant IPD has significantly increased since 2016 (4.2%-9.5%, p<0.05).
Conclusion: Though the incidence of IPD decreased in 2020 in comparison to previous years across all age groups, disease due to PCV13 serotypes 3 and 4, as well as non-PCV13 serotypes such as 8 and 12F, increased in prevalence. Continued surveillance of IPD is imperative to monitor shifts in serotype distribution and antimicrobial resistance.
Keywords: Canada; IPD; PCV13; Streptococcus pneumoniae; antimicrobial resistance; invasive pneumococcal disease; pneumococcus; serotype; surveillance.
Conflict of interest statement
Competing interests None.
Figures

References
-
- Ganaie F, Saad JS, McGee L, van Tonder AJ, Bentley SD, Lo SW, Gladstone RA, Turner P, Keenan JD, Breiman RF, Nahm MH. A new pneumococcal capsule type, 10D, is the 100th serotype and has a large cps fragment from an oral Streptococcus. MBio 2020;11(3):e00937–20. 10.1128/mBio.00937-20 - DOI - PMC - PubMed
-
- Bettinger JA, Scheifele DW, Kellner JD, Halperin SA, Vaudry W, Law B, Tyrrell G; Canadian Immunization Monitoring Program, Active (IMPACT) . The effect of routine vaccination on invasive pneumococcal infections in Canadian children, Immunization Monitoring Program, Active 2000-2007. Vaccine 2010;28(9):2130–6. 10.1016/j.vaccine.2009.12.026 - DOI - PubMed
-
- Demczuk WH, Martin I, Griffith A, Lefebvre B, McGeer A, Shane A, Zhanel GG, Tyrrell GJ, Gilmour MW; Toronto Invasive Bacterial Diseases Network; Canadian Public Health Laboratory Network . Serotype distribution of invasive Streptococcus pneumoniae in Canada during the introduction of the 13-valent pneumococcal conjugate vaccine, 2010. Can J Microbiol 2012;58(8):1008–17. 10.1139/w2012-073 - DOI - PubMed
-
- Desai S, McGeer A, Quach-Thanh C, Elliott D; approved by NACI. . Update on the Use of Conjugate Pneumococcal Vaccines in Childhood: An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI)†. Can Commun Dis Rep 2010;36 ACS-12:1–21. 10.14745/ccdr.v36i00a12 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
