Implementation of a mobile health clinic framework for Hepatitis C virus screening and treatment: a descriptive study
- PMID: 38124995
- PMCID: PMC10733089
- DOI: 10.1016/j.lana.2023.100648
Implementation of a mobile health clinic framework for Hepatitis C virus screening and treatment: a descriptive study
Abstract
Background: Although treatment for Hepatitis C Virus (HCV) is effective, individuals face access barriers. The utility of mobile health clinics (MHC), effective mechanisms for providing healthcare to underserved populations, is understudied for HCV-related interventions. We aimed to describe implementation of, and factors associated with, screening and treatment via MHCs.
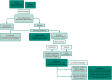
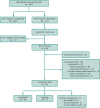
Methods: Clemson Rural Health implemented a novel MHC program to reach and treat populations at-risk for HCV with a focus on care for uninsured individuals. We examined HCV screening and treatment initiation/completion indicators between May 2021 and January 2023.
Findings: Among 607 individuals screened across 31 locations, 94 (15.5%) tested positive via antibody and viral load testing. Treatment initiation and completion rates were 49.6% and 86.0%, respectively. Among those screened, the majority were male (57.5%), White (61.3%; Black/Hispanic: 28.2%/7.7%), and without personal vehicle as primary transportation mode (54.4%). Injection drug use (IDU) was 27.2% and uninsured rate was 42.8%. Compared to HCV-negative, those infected included more individuals aged 30-44 (52.1% vs. 36.4%, p = 0.023), male (70.2% vs. 55.2%, p = 0.009), White (78.5% vs. 60.2%, p < 0.0001), without personal vehicle (58.5% vs. 43.5%, p = 0.028), IDU (83.7% vs. 21.0%, p < 0.0001), and uninsured (61.2% vs. 48.8%, p = 0.050). Uninsured rates were higher among those initiating compared to not initiating treatment (74.5% vs. 45.3%, p = 0.004).
Interpretation: The MHC framework successfully reaching its target population: at-risk individuals with access barriers to healthcare. The high HCV screening and treatment initiation/completion rates demonstrate the utility of MHCs as effective and acceptable intervention settings among historically difficult-to-treat populations.
Funding: Gilead Sciences, Inc., and SC Center for Rural and Primary Healthcare.
Keywords: Hepatitis C virus; Mobile health clinics; People who inject drugs; Underserved populations; Uninsured populations.
© 2023 The Author(s).
Conflict of interest statement
The program was supported by Gilead Sciences, Inc., (IN-US-987-5892) with additional funding specifically for uninsured patients from South Carolina Center for Rural and Primary Healthcare (2015593). KB, RG, CMK, PR, and AHL received support from South Carolina Center for Rural and Primary Healthcare during this study. KB, CMK, and AC received support from Gilead Sciences, Inc., during this study. KB received support for leading a round table discussion at a conference from International Network on Health and Hepatitis in Substance Users, speaking on a panel at a conference from Gilead Sciences, Inc., and travel to a conference from International Network on Health and Hepatitis in Substance Users. LR and FG received support from the National Library of Medicine of the National Institutes of Health (R01 LM014193-01A1) during this study. The funders had no role in the design, conduct, reporting of the study, or decision to submit for publication. Payments were made to our institution. AHL has served on the advisory boards for Gilead Sciences, Inc., and AbbVie.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

