Relationship among α‑synuclein, aging and inflammation in Parkinson's disease (Review)
- PMID: 38125364
- PMCID: PMC10728906
- DOI: 10.3892/etm.2023.12311
Relationship among α‑synuclein, aging and inflammation in Parkinson's disease (Review)
Abstract
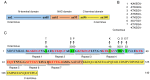
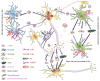
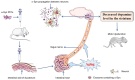
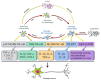
Parkinson's disease (PD) is a common neurodegenerative pathology whose major clinical symptoms are movement disorders. The main pathological characteristics of PD are the selective death of dopaminergic (DA) neurons in the pars compacta of the substantia nigra and the presence of Lewy bodies containing α-synuclein (α-Syn) within these neurons. PD is associated with numerous risk factors, including environmental factors, genetic mutations and aging. In many cases, the complex interplay of numerous risk factors leads to the onset of PD. The mutated α-Syn gene, which expresses pathologicalα-Syn protein, can cause PD. Another important feature of PD is neuroinflammation, which is conducive to neuronal death. α-Syn is able to interact with certain cell types in the brain, including through phagocytosis and degradation of α-Syn by glial cells, activation of inflammatory pathways by α-Syn in glial cells, transmission of α-Syn between glial cells and neurons, and interactions between peripheral immune cells and α-Syn. In addition to the aforementioned risk factors, PD may also be associated with aging, as the prevalence of PD increases with advancing age. The aging process impairs the cellular clearance mechanism, which leads to chronic inflammation and the accumulation of intracellular α-Syn, which results in DA neuronal death. In the present review, the age-associated α-Syn pathogenicity and the interactions between α-Syn and certain types of cells within the brain are discussed to facilitate understanding of the mechanisms of PD pathogenesis, which may potentially provide insight for the future clinical treatment of PD.
Keywords: Parkinson's disease; aging; alpha-synuclein; astrocytes; dopaminergic neurons; microglia; neurodegenerative disorder; neuroinflammation; oligodendrocytes; peripheral immune cells.
Copyright: © Zhang et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Association of Glial Activation and α-Synuclein Pathology in Parkinson's Disease.Neurosci Bull. 2023 Mar;39(3):479-490. doi: 10.1007/s12264-022-00957-z. Epub 2022 Oct 14. Neurosci Bull. 2023. PMID: 36229715 Free PMC article. Review.
-
Age-dependent neurodegeneration and neuroinflammation in a genetic A30P/A53T double-mutated α-synuclein mouse model of Parkinson's disease.Neurobiol Dis. 2022 Sep;171:105798. doi: 10.1016/j.nbd.2022.105798. Epub 2022 Jun 21. Neurobiol Dis. 2022. PMID: 35750147
-
Inhibition of the JAK/STAT Pathway Protects Against α-Synuclein-Induced Neuroinflammation and Dopaminergic Neurodegeneration.J Neurosci. 2016 May 4;36(18):5144-59. doi: 10.1523/JNEUROSCI.4658-15.2016. J Neurosci. 2016. PMID: 27147665 Free PMC article.
-
Allelic difference in Mhc2ta confers altered microglial activation and susceptibility to α-synuclein-induced dopaminergic neurodegeneration.Neurobiol Dis. 2017 Oct;106:279-290. doi: 10.1016/j.nbd.2017.07.016. Epub 2017 Jul 20. Neurobiol Dis. 2017. PMID: 28736195
-
Modeling Parkinson's Disease With the Alpha-Synuclein Protein.Front Pharmacol. 2020 Apr 23;11:356. doi: 10.3389/fphar.2020.00356. eCollection 2020. Front Pharmacol. 2020. PMID: 32390826 Free PMC article. Review.
Cited by
-
Potential common pathogenesis of several neurodegenerative diseases.Neural Regen Res. 2026 Mar 1;21(3):972-988. doi: 10.4103/NRR.NRR-D-24-01054. Epub 2025 May 30. Neural Regen Res. 2026. PMID: 40522761 Free PMC article.
-
Primary cilia in Parkinson's disease: summative roles in signaling pathways, genes, defective mitochondrial function, and substantia nigra dopaminergic neurons.Front Aging Neurosci. 2024 Sep 18;16:1451655. doi: 10.3389/fnagi.2024.1451655. eCollection 2024. Front Aging Neurosci. 2024. PMID: 39364348 Free PMC article. Review.
-
Shikonin Ameliorates Rotenone-Induced Neurotoxicity Through Inhibition of Apoptosis via IGF-1R/PI3K/AKT Pathway in a Parkinson's Disease-Associated SH-SY5Y Cell Model.Mol Neurobiol. 2025 Jul;62(7):8912-8930. doi: 10.1007/s12035-025-04810-y. Epub 2025 Mar 8. Mol Neurobiol. 2025. PMID: 40056341
-
Retinoid X Receptor as a Therapeutic Target to Treat Neurological Disorders Associated with α-Synucleinopathy.Cells. 2025 May 9;14(10):685. doi: 10.3390/cells14100685. Cells. 2025. PMID: 40422188 Free PMC article.
-
The potential role of creatine supplementation in neurodegenerative diseases.Phys Act Nutr. 2023 Dec;27(4):48-54. doi: 10.20463/pan.2023.0037. Epub 2023 Dec 31. Phys Act Nutr. 2023. PMID: 38297476 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
