Antiviral functionalization of cellulose using tannic acid and tannin-rich extracts
- PMID: 38125579
- PMCID: PMC10731304
- DOI: 10.3389/fmicb.2023.1287167
Antiviral functionalization of cellulose using tannic acid and tannin-rich extracts
Abstract
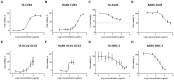
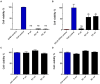
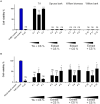
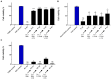
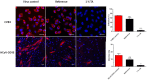
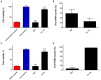
Due to seasonally appearing viruses and several outbreaks and present pandemic, we are surrounded by viruses in our everyday life. In order to reduce viral transmission, functionalized surfaces that inactivate viruses are in large demand. Here the endeavor was to functionalize cellulose-based materials with tannic acid (TA) and tannin-rich extracts by using different binding polymers to prevent viral infectivity of both non-enveloped coxsackievirus B3 (CVB3) and enveloped human coronavirus OC43 (HCoV-OC43). Direct antiviral efficacy of TA and spruce bark extract in solution was measured: EC50 for CVB3 was 0.12 and 8.41 μg/ml and for HCoV-OC43, 78.16 and 95.49 μg/ml, respectively. TA also led to an excellent 5.8- to 7-log reduction of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus infectivity. TA functionalized materials reduced infectivity already after 5-min treatment at room temperature. All the tested methods to bind TA showed efficacy on paperboard with 0.1 to 1% (w/v) TA concentrations against CVB3 whereas material hydrophobicity decreased activities. Specific signatures for TA and HCoV-OC43 were discovered by Raman spectroscopy and showed clear co-localization on the material. qPCR study suggested efficient binding of CVB3 to the TA functionalized cellulose whereas HCoV-OC43 was flushed out from the surfaces more readily. In conclusion, the produced TA-materials showed efficient and broadly acting antiviral efficacy. Additionally, the co-localization of TA and HCoV-OC43 and strong binding of CVB3 to the functionalized cellulose demonstrates an interaction with the surfaces. The produced antiviral surfaces thus show promise for future use to increase biosafety and biosecurity by reducing pathogen persistence.
Keywords: Raman spectroscopy; antiviral functionalization; bark extract; cellulose; coronaviruses; enteroviruses; tannic acid.
Copyright © 2023 Haapakoski, Emelianov, Reshamwala, Laajala, Tienaho, Kilpeläinen, Liimatainen, Jyske, Pettersson and Marjomäki.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Abdelkhalek A., Qari S. H., Abu-Saied M. A. A.-R., Khalil A. M., Younes H. A., Nehela Y., et al. (2021). Chitosan nanoparticles inactivate alfalfa mosaic virus replication and boost innate immunity in Nicotiana glutinosa plants. Plants 10:2701. doi: 10.3390/plants10122701, PMID: - DOI - PMC - PubMed
-
- Amankwaah C., Li J., Lee J., Pascall M. A. (2020). Development of antiviral and bacteriostatic chitosan-based food packaging material with grape seed extract for murine norovirus, Escherichia coli and Listeria innocua control. Food Sci. Nutr. 8, 6174–6181. doi: 10.1002/fsn3.1910 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

