Leveraging immunoliposomes as nanocarriers against SARS-CoV-2 and its emerging variants
- PMID: 38125653
- PMCID: PMC10730353
- DOI: 10.1016/j.ajps.2023.100855
Leveraging immunoliposomes as nanocarriers against SARS-CoV-2 and its emerging variants
Abstract
The global COVID-19 pandemic arising from SARS-CoV-2 has impacted many lives, gaining interest worldwide ever since it was first identified in December 2019. Till 2023, 752 million cumulative cases and 6.8 million deaths were documented globally. COVID-19 has been rapidly evolving, affecting virus transmissibility and properties and contributing to increased disease severity. The Omicron is the most circulating variant of concern. Although success in its treatment has indicated progress in tackling the virus, limitations in delivering the current antiviral agents in battling emerging variants remain remarkable. With the latest advancements in nanotechnology for controlling infectious diseases, liposomes have the potential to counteract SARS-CoV-2 because of their ability to employ different targeting strategies, incorporating monoclonal antibodies for the active and passive targeting of infected patients. This review will present a concise summary of the possible strategies for utilizing immunoliposomes to improve current treatment against the occurrence of SARS-CoV-2 and its variants.
Keywords: COVID-19; Coronavirus; Immunoliposomes; Liposomes; SARS-CoV-2.
© 2023 Shenyang Pharmaceutical University. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no conflicts of interest or financial competing interests.
Figures




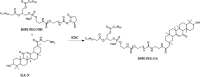
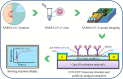
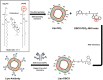
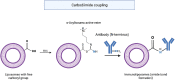
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

