Acoustic Molecular Imaging Beyond the Diffraction Limit In Vivo
- PMID: 38125957
- PMCID: PMC10732349
- DOI: 10.1109/ojuffc.2022.3212342
Acoustic Molecular Imaging Beyond the Diffraction Limit In Vivo
Abstract
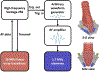
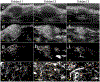
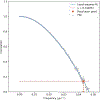
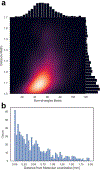
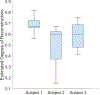
Ultrasound molecular imaging (USMI) is a technique used to noninvasively estimate the distribution of molecular markers in vivo by imaging microbubble contrast agents (MCAs) that have been modified to target receptors of interest on the vascular endothelium. USMI is especially relevant for preclinical and clinical cancer research and has been used to predict tumor malignancy and response to treatment. In the last decade, methods that improve the resolution of contrast-enhanced ultrasound by an order of magnitude and allow researchers to noninvasively image individual capillaries have emerged. However, these approaches do not translate directly to molecular imaging. In this work, we demonstrate super-resolution visualization of biomarker expression in vivo using superharmonic ultrasound imaging (SpHI) with dual-frequency transducers, targeted contrast agents, and localization microscopy processing. We validate and optimize the proposed method in vitro using concurrent optical and ultrasound microscopy and a microvessel phantom. With the same technique, we perform a proof-of-concept experiment in vivo in a rat fibrosarcoma model and create maps of biomarker expression co-registered with images of microvasculature. From these images, we measure a resolution of 23 μm, a nearly fivefold improvement in resolution compared to previous diffraction-limited molecular imaging studies.
Keywords: Molecular imaging; superharmonic imaging; ultrasound; ultrasound contrast agents; ultrasound localization microscopy.
Conflict of interest statement
CONFLICTS OF INTEREST F. Stuart Foster and Paul A. Dayton are inventors on a patent describing dual-frequency imaging, which is now licensed to Perkin Elmer. Thomas M. Kierski, Isabel G. Newsome, Gianmarco F. Pinton, and Paul A. Dayton are inventors on a patent describing dual-frequency ultrasound localization microscopy.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
