Potential application of bisoprolol derivative compounds as antihypertensive drugs: synthesis and in silico study
- PMID: 38126063
- PMCID: PMC10731320
- DOI: 10.1098/rsos.231112
Potential application of bisoprolol derivative compounds as antihypertensive drugs: synthesis and in silico study
Abstract
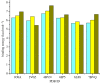
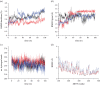
Two bisoprolol derivatives, N-acetyl bisoprolol and N-formyl bisoprolol, belonging to the beta-blocker class of antihypertensive drugs, were synthesized using acetylation and formylation reactions. The yields of the reactions were determined to be 32.40% for N-acetyl bisoprolol and 20.20% for N-formyl bisoprolol. In silico methods such as molecular docking, molecular dynamics simulation and SwissADME prediction were employed to evaluate the potential of these bisoprolol derivatives as antihypertensive drugs. These methods were used to assess the interaction between the bisoprolol derivatives and various receptors associated with hypertension, including human angiotensin I-converting enzyme (PDB ID: 1O8A), renin (PDB ID: 2V0Z), beta-1 adrenergic receptors (PDB ID: 4BVN, 7BVQ), voltage-dependent L-type calcium channel subunit alpha-1S (PDB ID: 6JP5) and mineralocorticoid receptor (PDB ID: 6L88). Our results demonstrated the highest binding energy when bisoprolol and its derivatives bound to 4BVN, with binding energy values of 6.74 kcal mol-1, 7.03 kcal mol-1 and 7.63 kcal mol-1 for bisoprolol, N-acetyl bisoprolol and N-formyl bisoprolol, respectively. The stability of these complexes was confirmed by molecular dynamics simulations, with a root-mean-square deviation value of approximately 2. Furthermore, the SwissADME results indicated that both derivatives exhibited similar properties to the reference drug bisoprolol.
Keywords: antihypertensives; bisoprolol; derivative; molecular docking; organic synthesis.
© 2023 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures

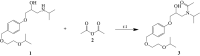
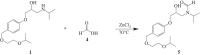
References
-
- Baba WN, Baby B, Mudgil P, Gan CY, Vijayan R, Maqsood S. 2021. Pepsin generated camel whey protein hydrolysates with potential antihypertensive properties: identification and molecular docking of antihypertensive peptides. LWT 143, 111135. (10.1016/j.lwt.2021.111135) - DOI
Associated data
LinkOut - more resources
Full Text Sources

