Requirement of Site-Specific Tyrosine Phosphorylation of Cortactin in Retinal Neovascularization and Vascular Leakage
- PMID: 38126170
- PMCID: PMC10872470
- DOI: 10.1161/ATVBAHA.123.320279
Requirement of Site-Specific Tyrosine Phosphorylation of Cortactin in Retinal Neovascularization and Vascular Leakage
Abstract
Background: Retinal neovascularization is a major cause of vision impairment. Therefore, the purpose of this study is to investigate the mechanisms by which hypoxia triggers the development of abnormal and leaky blood vessels.
Methods: A variety of cellular and molecular approaches as well as tissue-specific knockout mice were used to investigate the role of Cttn (cortactin) in retinal neovascularization and vascular leakage.
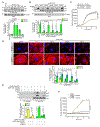
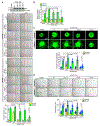
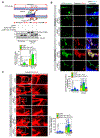
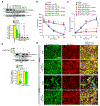
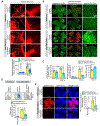
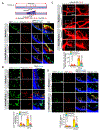
Results: We found that VEGFA (vascular endothelial growth factor A) stimulates Cttn phosphorylation at Y421, Y453, and Y470 residues in human retinal microvascular endothelial cells. In addition, we observed that while blockade of Cttn phosphorylation at Y470 inhibited VEGFA-induced human retinal microvascular endothelial cell angiogenic events, suppression of Y421 phosphorylation protected endothelial barrier integrity from disruption by VEGFA. In line with these observations, while blockade of Cttn phosphorylation at Y470 negated oxygen-induced retinopathy-induced retinal neovascularization, interference with Y421 phosphorylation prevented VEGFA/oxygen-induced retinopathy-induced vascular leakage. Mechanistically, while phosphorylation at Y470 was required for its interaction with Arp2/3 and CDC6 facilitating actin polymerization and DNA synthesis, respectively, Cttn phosphorylation at Y421 leads to its dissociation from VE-cadherin, resulting in adherens junction disruption. Furthermore, whereas Cttn phosphorylation at Y470 residue was dependent on Lyn, its phosphorylation at Y421 residue required Syk activation. Accordingly, lentivirus-mediated expression of shRNA targeting Lyn or Syk levels inhibited oxygen-induced retinopathy-induced retinal neovascularization and vascular leakage, respectively.
Conclusions: The above observations show for the first time that phosphorylation of Cttn is involved in a site-specific manner in the regulation of retinal neovascularization and vascular leakage. In view of these findings, Cttn could be a novel target for the development of therapeutics against vascular diseases such as retinal neovascularization and vascular leakage.
Keywords: cortactin; endothelial cells; neovascularization; vascular endothelial growth factor; vascular leakage.
Conflict of interest statement
Figures




Similar articles
-
Novel Role of Prereplication Complex Component Cell Division Cycle 6 in Retinal Neovascularization.Arterioscler Thromb Vasc Biol. 2022 Apr;42(4):407-427. doi: 10.1161/ATVBAHA.121.317182. Epub 2022 Mar 3. Arterioscler Thromb Vasc Biol. 2022. PMID: 35236105 Free PMC article.
-
Glucose-Regulated Protein 78, via Releasing β-Catenin from Adherens Junctions, Facilitates Its Interaction with STAT3 in Mediating Retinal Neovascularization.Am J Pathol. 2024 Dec;194(12):2356-2381. doi: 10.1016/j.ajpath.2024.08.005. Epub 2024 Aug 31. Am J Pathol. 2024. PMID: 39222910
-
eNOS-induced vascular barrier disruption in retinopathy by c-Src activation and tyrosine phosphorylation of VE-cadherin.Elife. 2021 Apr 28;10:e64944. doi: 10.7554/eLife.64944. Elife. 2021. PMID: 33908348 Free PMC article.
-
Cortactin in Lung Cell Function and Disease.Int J Mol Sci. 2022 Apr 21;23(9):4606. doi: 10.3390/ijms23094606. Int J Mol Sci. 2022. PMID: 35562995 Free PMC article. Review.
-
[Cell biology of intraocular vascular diseases].Nippon Ganka Gakkai Zasshi. 1999 Dec;103(12):923-47. Nippon Ganka Gakkai Zasshi. 1999. PMID: 10643294 Review. Japanese.
Cited by
-
Alpha-1 antitrypsin reduces inflammation and vasculopathy in mice with oxygen-induced retinopathy.J Inflamm (Lond). 2025 Feb 11;22(1):6. doi: 10.1186/s12950-025-00431-3. J Inflamm (Lond). 2025. PMID: 39934776 Free PMC article.
References
-
- Peto T, Resnikoff S, Kempen JH, Steinmetz JD, Briant PS, Wong TY, Friedman DS, Bron AM, Jonas J, Fernandes A, et al. Diabetic retinopathy contributes to global vision loss. Invest. Ophthalmol. Vis. Sci 2021;62:1139.
-
- World Health Organization. Regional Office for South-East Asia. Strengthening diagnosis and treatment of Diabetic Retinopathy in SEA Region. 2020; Available at: https://apps.who.int/iris/handle/10665/334224 (accessed Apr 17, 2023).
-
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2020; Available at: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-stat... (accessed Apr 17, 2023).
-
- International Diabetes Federation. IDF Diabetes Atlas, 10th edn. Brussels, Belgium: 2021; Available at: https://www.diabetesatlas.org (accessed Apr 17, 2023).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

