Urine tenofovir and dried blood spot tenofovir diphosphate concentrations and viraemia in people taking efavirenz and dolutegravir-based antiretroviral therapy
- PMID: 38126342
- PMCID: PMC7615742
- DOI: 10.1097/QAD.0000000000003818
Urine tenofovir and dried blood spot tenofovir diphosphate concentrations and viraemia in people taking efavirenz and dolutegravir-based antiretroviral therapy
Abstract
Objective: We aimed to determine whether urine tenofovir (TFV) and dried blood spot (DBS) tenofovir diphosphate (TFV-DP) concentrations are associated with concurrent HIV viraemia.
Design: Cross-sectional study among people with HIV (PWH) receiving tenofovir disoproxil fumarate (TDF)-based antiretroviral therapy (ART).
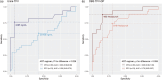
Methods: We used dual tandem liquid chromatography and mass spectrometry to measure urine TFV and DBS TFV-DP concentrations, and evaluated their associations with concurrent viraemia at least 1000 copies/ml using logistic regression models. In exploratory analyses, we used receiver operating curves (ROCs) to estimate optimal urine TFV and DBS TFV-DP thresholds to predict concurrent viraemia.
Results: Among 124 participants, 68 (54.8%) were women, median age was 39 years [interquartile range (IQR) 34-45] and 74 (59.7%) were receiving efavirenz versus 50 (40.3%) receiving dolutegravir. Higher concentrations of urine TFV [1000 ng/ml increase, odds ratio (OR) 0.97 95% CI 0.94-0.99, P = 0.005] and DBS TFV-DP (100 fmol/punch increase, OR 0.76, 95% CI 0.67-0.86, P < 0.001) were associated with lower odds of viraemia. There was evidence that these associations were stronger among people receiving dolutegravir than among people receiving efavirenz (urine TFV, P = 0.072; DBS TFV-DP, P = 0.003). Nagelkerke pseudo- R2 for the DBS TFV-DP models was higher for the urine TFV models, demonstrating a stronger relationship between DBS TFV-DP and viraemia. Among people receiving dolutegravir, a DBS TFV-DP concentration of 483 fmol/punch had 88% sensitivity and 85% specificity to predict concurrent viraemia ≥1000 copies/ml.
Conclusion: Among PWH receiving TDF-based ART, urine TFV concentrations, and in particular DBS TFV-DP concentrations, were strongly associated with concurrent viraemia, especially among people receiving dolutegravir.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
There are no conflicts of interest.
Figures
Similar articles
-
A Comparison of Plasma Efavirenz and Tenofovir, Dried Blood Spot Tenofovir-Diphosphate, and Self-Reported Adherence to Predict Virologic Suppression Among South African Women.J Acquir Immune Defic Syndr. 2019 Jul 1;81(3):311-318. doi: 10.1097/QAI.0000000000002032. J Acquir Immune Defic Syndr. 2019. PMID: 30893125 Free PMC article.
-
Diagnostic accuracy of a point-of-care urine tenofovir assay, and associations with HIV viraemia and drug resistance among people receiving dolutegravir and efavirenz-based antiretroviral therapy.J Int AIDS Soc. 2023 Sep;26(9):e26172. doi: 10.1002/jia2.26172. J Int AIDS Soc. 2023. PMID: 37735860 Free PMC article.
-
Relationship Between Tenofovir Diphosphate Concentrations in Dried Blood Spots and Virological Outcomes After Initiating Tenofovir-Lamivudine-Dolutegravir as First-Line or Second-Line Antiretroviral Therapy.J Acquir Immune Defic Syndr. 2024 Mar 1;95(3):260-267. doi: 10.1097/QAI.0000000000003341. J Acquir Immune Defic Syndr. 2024. PMID: 38408216 Free PMC article.
-
Tenofovir-Diphosphate and Emtricitabine-Triphosphate Adherence Benchmarks in Dried Blood Spots for Persons With HIV Receiving Tenofovir Alafenamide and Emtricitabine-Based Antiretroviral Therapy (QUANTI-TAF).Clin Infect Dis. 2024 Nov 22;79(5):1233-1241. doi: 10.1093/cid/ciae212. Clin Infect Dis. 2024. PMID: 38636950 Free PMC article.
-
Measurements of tenofovir-diphosphate and emtricitabine-triphosphate concentrations in dried blood spots of people receiving pre-exposure prophylaxis for HIV with co-formulated tenofovir disoproxil fumarate and emtricitabine.J Microbiol Immunol Infect. 2025 Aug;58(4):414-421. doi: 10.1016/j.jmii.2025.03.002. Epub 2025 Mar 8. J Microbiol Immunol Infect. 2025. PMID: 40087092
Cited by
-
Recommendations for interpreting intraerythrocytic tenofovir-diphosphate and emtricitabine-triphosphate for PrEP adherence assessment.J Acquir Immune Defic Syndr. 2025 Jun 19:10.1097/QAI.0000000000003715. doi: 10.1097/QAI.0000000000003715. Online ahead of print. J Acquir Immune Defic Syndr. 2025. PMID: 40536250 Free PMC article.
-
HIV drug resistance, early treatment outcomes and impact of guidelines compliance after protease inhibitor-based second-line failure in a dedicated resistance clinic in western Kenya: a retrospective cohort study.J Int AIDS Soc. 2025 Jun;28(6):e26523. doi: 10.1002/jia2.26523. J Int AIDS Soc. 2025. PMID: 40490983 Free PMC article.
References
-
- World Health Organization. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. 2021, World Health Organization: Geneva, Switzerland. - PubMed
-
- Clinton Health Access Initiative. 2021 HIV Market Report: The state of the HIV market in low- and middle-income countries. 2021, Clinto Health Access Initiative. Boston, USA.
-
- Barditch-Crovo P, Deeks SG, Collier A, Safrin S, Coakley DF, Miller M, et al. . Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother 2001; 45:2733–2739. - PMC - PubMed
-
- Hermans LE, Umunnakwe CN, Lalla-Edward ST, Hebel SK, Tempelman HA, Nijhuis M, et al. . Point-of-care tenofovir urine testing for the prediction of treatment failure and drug resistance during initial treatment for human immunodeficiency virus type 1 (HIV-1) infection. Clin Infect Dis 2023; 76:e553–e560. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical