Intensive care risk and long-term outcomes in pediatric allogeneic hematopoietic cell transplant recipients
- PMID: 38127268
- PMCID: PMC10879681
- DOI: 10.1182/bloodadvances.2023011002
Intensive care risk and long-term outcomes in pediatric allogeneic hematopoietic cell transplant recipients
Abstract
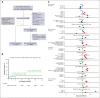
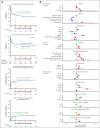
Allogeneic hematopoietic cell transplantation (HCT) can be complicated by life-threatening organ toxicity and infection necessitating intensive care. Epidemiologic data have been limited by single-center studies, poor database granularity, and a lack of long-term survivors. To identify contemporary trends in intensive care unit (ICU) use and long-term outcomes, we merged data from the Center for International Blood and Marrow Transplant Research and the Virtual Pediatric Systems databases. We identified 6995 pediatric patients with HCT aged ≤21 years who underwent first allogeneic HCT between 2008 and 2014 across 69 centers in the United States or Canada and followed patients until the year 2020. ICU admission was required for 1067 patients (8.3% by day +100, 12.8% by 1 year, and 15.3% by 5 years after HCT), and was linked to demographic background, pretransplant organ toxicity, allograft type and HLA-match, and the development of graft-versus-host disease or malignancy relapse. Survival to ICU discharge was 85.7%, but more than half of ICU survivors required ICU readmission, leading to 52.5% and 42.6% survival at 1- and 5-years post-ICU transfer, respectively. ICU survival was worse among patients with malignant disease, poor pretransplant organ function, and alloreactivity risk factors. Among 1-year HCT survivors, those who required ICU in the first year had 10% lower survival at 5 years and developed new dialysis-dependent renal failure at a greater rate (P<.001). Thus, although ICU management is common and survival to ICU discharge is high, ongoing complications necessitate recurrent ICU admission and lead to a poor 1-year outcome in select patients who are at high risk.
Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution.
Conflict of interest statement
Conflict of interest disclosure: A.S. has received consultant fee from Spotlight Therapeutics, Medexus Inc, Vertex Pharmaceuticals, Sangamo Therapeutics and Editas Medicine. He is a medical monitor for RCI BMT CSIDE clinical trial for which he receives financial compensation. He has also received research funding from CRISPR Therapeutics and honoraria from Vindico Medical Education. A.S. is the St. Jude Children’s Research Hospital–site principal investigator of clinical trials for genome editing of sickle cell disease sponsored by Vertex Pharmaceuticals/CRISPR Therapeutics (NCT03745287), Novartis Pharmaceuticals (NCT04443907) and Beam Therapeutics (NCT05456880). The industry sponsors provide funding for the clinical trial, which includes salary support paid to A.S.’s institution. A.S. has no direct financial interest in these therapies. G.G. is the Principal Investigator of Project Sickle Cure, a Sickle Cell Transplant Advocacy and Research Alliance (STAR) study partially funded by bluebird bio. H.S. reports having received personal fees from Incyte, Janssen, Novartis, Sanofi and from the Belgian Hematological Society (BHS), as well as research grants from Novartis and the BHS, all paid to her institution and not related to this work. H.S. has also received nonfinancial support (travel grants) from Gilead, Pfizer, the European Society for Blood and Marrow Transplantation and the Center for International Bone Marrow Transplantation Research. M.S. served as a consultant and received honoraria from Jazz Pharmaceutical Canada. He received research funding from National Cancer Institute, PCORI, and Bluenote. C.C.D. reports consulting with Jazz Pharma and Alexion Inc. R.P. reports compensation from bluebird bio (advisory board) and Amgen (research funding; ended December 2021). K.M. reports significant payments: investigator initiated clinical trial sponsored by Incyte for early intervention for pulmonary dysfunction after stem cell transplant with ruxolitinib and industry sponsored clinical trial for ex vivo telomere elongation for patients with telomere biology disorders by Elixirgen Therapeutics and Proprietary Interests. K.M. has filed US provisional patent application #63/061,334, on 5 August 2021, entitled “Composition and Methods for the Treatment of Bronchiolitis Obliterans.” T.N. reports significant payments: research support to the institution for clinical trial (Novartis), research support (drug supply only) to the institution for clinical trial (Karyopharm) and relationships, advisory board membership (Medexus). The remaining authors report no competing financial interests.
Figures
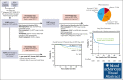

Update of
-
Critical Illness Risk and Long-Term Outcomes Following Intensive Care in Pediatric Hematopoietic Cell Transplant Recipients.medRxiv [Preprint]. 2023 Aug 5:2023.07.31.23293444. doi: 10.1101/2023.07.31.23293444. medRxiv. 2023. Update in: Blood Adv. 2024 Feb 27;8(4):1002-1017. doi: 10.1182/bloodadvances.2023011002. PMID: 37577706 Free PMC article. Updated. Preprint.
References
-
- Kaya Z, Weiner DJ, Yilmaz D, Rowan J, Goyal RK. Lung function, pulmonary complications, and mortality after allogeneic blood and marrow transplantation in children. Biol Blood Marrow Transplant. 2009;15(7):817–826. - PubMed
-
- Zinter MS, Logan BR, Fretham C, et al. Comprehensive prognostication in critically ill pediatric hematopoietic cell transplant patients: results from Merging the Center for International Blood and Marrow Transplant Research (CIBMTR) and Virtual Pediatric Systems (VPS) Registries. Biol Blood Marrow Transplant. 2020;26(2):333–342. - PMC - PubMed

