Race and Ethnicity of Infants Enrolled in Neonatal Clinical Trials: A Systematic Review
- PMID: 38127349
- PMCID: PMC10739112
- DOI: 10.1001/jamanetworkopen.2023.48882
Race and Ethnicity of Infants Enrolled in Neonatal Clinical Trials: A Systematic Review
Abstract
Importance: Representativeness of populations within neonatal clinical trials is crucial to moving the field forward. Although racial and ethnic disparities in research inclusion are well documented in other fields, they are poorly described within neonatology.
Objective: To describe the race and ethnicity of infants included in a sample of recent US neonatal clinical trials and the variability in this reporting.
Evidence review: A systematic search of US neonatal clinical trials entered into Cochrane CENTRAL 2017 to 2021 was conducted. Two individuals performed inclusion determination, data extraction, and quality assessment independently with discrepancies adjudicated by consensus.
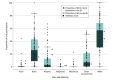
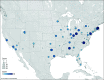
Findings: Of 120 studies with 14 479 participants that met the inclusion criteria, 75 (62.5%) included any participant race or ethnicity data. In the studies that reported race and ethnicity, the median (IQR) percentage of participants of each background were 0% (0%-1%) Asian, 26% (9%-42%) Black, 3% (0%-12%) Hispanic, 0% (0%-0%) Indigenous (eg, Alaska Native, American Indian, and Native Hawaiian), 0% (0%-0%) multiple races, 57% (30%-68%) White, and 7% (1%-21%) other race or ethnicity. Asian, Black, Hispanic, and Indigenous participants were underrepresented, while White participants were overrepresented compared with a reference sample of the US clinical neonatal intensive care unit (NICU) population from the Vermont Oxford Network. Many participants were labeled as other race or ethnicity without adequate description. There was substantial variability in terms and methods of reporting race and ethnicity data. Geographic representation was heavily skewed toward the Northeast, with nearly one-quarter of states unrepresented.
Conclusions and relevance: These findings suggest that neonatal research may perpetuate inequities by underrepresenting Asian, Black, Hispanic, and Indigenous neonates in clinical trials. Studies varied in documentation of race and ethnicity, and there was regional variation in the sites included. Based on these findings, funders and clinical trialists are advised to consider a 3-point targeted approach to address these issues: prioritize identifying ways to increase diversity in neonatal clinical trial participation, agree on a standardized method to report race and ethnicity among neonatal clinical trial participants, and prioritize the inclusion of participants from all regions of the US in neonatal clinical trials.
Conflict of interest statement
Figures
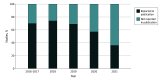
References
-
- Natale JE, Lebet R, Joseph JG, et al. ; Randomized Evaluation of Sedation Titration for Respiratory Failure (RESTORE) Study Investigators . Racial and ethnic disparities in parental refusal of consent in a large, multisite pediatric critical care clinical trial. J Pediatr. 2017;184:204-208.e1. doi: 10.1016/j.jpeds.2017.02.006 - DOI - PubMed
-
- Paquette E, Shukla A, Duyar S. Social Determinants of Research Engagement and Implications for Precision Medicine: Sociodemographic Factors Associated with Differential Enrollment in a Pediatric Critical Care Biorepository. Pediatric Academic Societies; 2018.
-
- Foglia EE, Nolen TL, DeMauro SB, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Short-term outcomes of infants enrolled in randomized clinical trials vs those eligible but not enrolled. JAMA. 2015;313(23):2377-2379. doi: 10.1001/jama.2015.5734 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

