Mechanosensitive channels TMEM63A and TMEM63B mediate lung inflation-induced surfactant secretion
- PMID: 38127458
- PMCID: PMC10904053
- DOI: 10.1172/JCI174508
Mechanosensitive channels TMEM63A and TMEM63B mediate lung inflation-induced surfactant secretion
Abstract
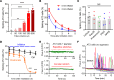
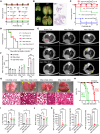
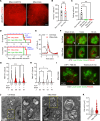
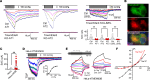
Pulmonary surfactant is a lipoprotein complex lining the alveolar surface to decrease the surface tension and facilitate inspiration. Surfactant deficiency is often seen in premature infants and in children and adults with respiratory distress syndrome. Mechanical stretch of alveolar type 2 epithelial (AT2) cells during lung expansion is the primary physiological factor that stimulates surfactant secretion; however, it is unclear whether there is a mechanosensor dedicated to this process. Here, we show that loss of the mechanosensitive channels TMEM63A and TMEM63B (TMEM63A/B) resulted in atelectasis and respiratory failure in mice due to a deficit of surfactant secretion. TMEM63A/B were predominantly localized at the limiting membrane of the lamellar body (LB), a lysosome-related organelle that stores pulmonary surfactant and ATP in AT2 cells. Activation of TMEM63A/B channels during cell stretch facilitated the release of surfactant and ATP from LBs fused with the plasma membrane. The released ATP evoked Ca2+ signaling in AT2 cells and potentiated exocytic fusion of more LBs. Our study uncovered a vital physiological function of TMEM63 mechanosensitive channels in preparing the lungs for the first breath at birth and maintaining respiration throughout life.
Keywords: Ion channels; Pulmonary surfactants; Pulmonology.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

