Emicizumab prophylaxis in infants with hemophilia A (HAVEN 7): primary analysis of a phase 3b open-label trial
- PMID: 38127586
- PMCID: PMC11033591
- DOI: 10.1182/blood.2023021832
Emicizumab prophylaxis in infants with hemophilia A (HAVEN 7): primary analysis of a phase 3b open-label trial
Abstract
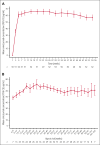
Subcutaneous emicizumab enables prophylaxis for people with hemophilia A (HA) from birth, potentially reducing risk of bleeding and intracranial hemorrhage (ICH). HAVEN 7 (NCT04431726) is the first clinical trial of emicizumab dedicated to infants, designed to investigate the efficacy, safety, pharmacokinetics, and pharmacodynamics of emicizumab in those aged ≤12 months with severe HA without factor VIII (FVIII) inhibitors. Participants in this phase 3b trial received emicizumab 3 mg/kg maintenance dose every 2 weeks for 52 weeks and are continuing emicizumab during the 7-year long-term follow-up. Efficacy end points included annualized bleed rate (ABR): treated, all, treated spontaneous, and treated joint bleeds. Safety end points included adverse events (AEs), thromboembolic events (TEs), thrombotic microangiopathies (TMAs), and immunogenicity (anti-emicizumab antibodies [ADAs] and FVIII inhibitors). At primary analysis, 55 male participants had received emicizumab (median treatment duration: 100.3; range, 52-118 weeks). Median age at informed consent was 4.0 months (range, 9 days to 11 months 30 days). Model-based ABR for treated bleeds was 0.4 (95% confidence interval, 0.30-0.63), with 54.5% of participants (n = 30) having zero treated bleeds. No ICH occurred. All 42 treated bleeds in 25 participants (45.5%) were traumatic. Nine participants (16.4%) had ≥1 emicizumab-related AE (all grade 1 injection-site reactions). No AE led to treatment changes. No deaths, TEs, or TMAs occurred. No participant tested positive for ADAs. Two participants were confirmed positive for FVIII inhibitors. This primary analysis of HAVEN 7 indicates that emicizumab is efficacious and well tolerated in infants with severe HA without FVIII inhibitors.
© 2024 American Society of Hematology. Published by Elsevier Inc. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Conflict-of-interest disclosure: S.W.P. is a member of the scientific advisory board for GeneVentiv and Equilibra Bioscience and has received grants or contracts from Siemens; consulting fees from Apcintex, ASC Therapeutics, Bayer, BioMarin, CSL Behring, HEMA Biologics, Freeline, LFB Group, Novo Nordisk, Pfizer, Regeneron/Intellia, Genentech, Inc./F. Hoffmann-La Roche Ltd, Sanofi, Takeda, Spark Therapeutics, and uniQure. P.C. is a member on an entity’s board of directors for the HAVEN 7 trial steering committee and a member of the UK Haemophilia Centre Doctors’ Organization, which has received a research grant from F. Hoffmann-La Roche Ltd. C.D. is an employee of F. Hoffmann-La Roche Ltd. G.K. has received grants/research support funding from Binational Science Foundation, Pfizer, F. Hoffmann-La Roche Ltd, Tel Aviv University, and Sheba research authorities; consulting fees from ASC Therapeutics, Bayer, BioMarin, Novo Nordisk, Pfizer, F. Hoffmann-La Roche Ltd, Sobi, Sanofi-Genzyme, Takeda, and uniQure; honoraria from Bayer, BioMarin, Bio Products Laboratory, CSL Behring, Pfizer, Novo Nordisk, F. Hoffmann-La Roche Ltd, Sanofi-Genzyme, and Spark Therapeutics; participated on a data safety monitoring board or advisory board for ASC Therapeutics, BioMarin, Pfizer, Novo Nordisk, uniQure, F. Hoffmann-La Roche Ltd, Sanofi-Genzyme, Sobi, and Spark Therapeutics; and has a leadership role in PedNet Research foundation. C.S. and M.B. are employees and stockholders of F. Hoffmann-La Roche Ltd. V.J.-Y. has received grants or contracts from F. Hoffmann-La Roche Ltd, Novo Nordisk, Sobi, Takeda, Grifols, Bayer, Pfizer, Octapharma, and CSL Behring; consulting fees from F. Hoffmann-La Roche Ltd, Novo Nordisk, Sanofi, Sobi, Takeda, Grifols, Bayer, Pfizer, Spark Therapeutics, BioMarin, Octapharma, and CSL Behring; and honoraria from F. Hoffmann-La Roche Ltd, Novo Nordisk, Sanofi, Sobi, Takeda, Grifols, Bayer, Pfizer, Spark Therapeutics, BioMarin, Octapharma, and CSL Behring. F.P. has received speaker fees for participating in advisory boards from Sanofi, Sobi, Takeda, F. Hoffmann-La Roche Ltd, and BioMarin; and educational meeting fees from Grifols and F. Hoffmann-La Roche Ltd. G.Y. has received grants or contracts from Genentech, Inc., Grifols, and Takeda; royalties or licenses from Viatris; consulting fees from Bayer, BioMarin, CSL Behring, Genentech, Inc./F. Hoffmann-La Roche Ltd, LFB Group, Novo Nordisk, Pfizer, Sanofi, Spark Therapeutics, and Takeda; honoraria from Bayer, BioMarin, CSL Behring, Genentech, Inc./F. Hoffmann-La Roche Ltd, Novo Nordisk, Pfizer, Sanofi, Spark Therapeutics, and Takeda; and speakers bureau fees from BioMarin, Genentech, Inc., Hema Biologics, Sanofi, and Spark Therapeutics. J.O. has received research funding from Bayer, Biotest, CSL Behring, Octapharma, Pfizer, Swedish Orphan Biovitrum, and Takeda; consultancy, speakers bureau fees, honoraria, scientific advisory board fees, and travel expenses from Bayer, Biogen Idec, BioMarin, Biotest, Chugai Pharmaceutical Co., Ltd, CSL Behring, Freeline, Grifols, LFB Group, Novo Nordisk, Octapharma, Pfizer, F. Hoffmann-La Roche Ltd, Sanofi, Spark Therapeutics, Swedish Orphan Biovitrum, and Takeda. M.E.M. has received consulting fees from Bayer, CSL Behring, Novo Nordisk, F. Hoffmann-La Roche Ltd, Octapharma, Pfizer, Sanofi, Sobi, Kedrion, Grifols, BioMarin, Catalyst, uniQure, and LFB Group; honoraria from Bayer, CSL Behring, Novo Nordisk, F. Hoffmann-La Roche Ltd, Octapharma, Pfizer, Sobi, Kedrion, Grifols, BioMarin, and Spark Therapeutics. K.K. has received consulting and honoraria fees from F. Hoffmann-La Roche Ltd, Novo Nordisk, Takeda, and Pfizer; and research funding from F. Hoffmann-La Roche Ltd and Pfizer. S.D. is an employee of Genentech, Inc. A.K., M.N., and M.L. are employees and have stock ownership in F. Hoffmann-La Roche Ltd. T.C is an employee of Spark Therapeutics, which is part of the Roche group and holds stock in F. Hoffmann-La Roche Ltd. The institution of K.F. has received unrestricted research grants from CSL Behring, Sobi, and Novo Nordisk; and consultancy fees from F. Hoffmann-La Roche Ltd, Sanofi, Sobi, and Novo Nordisk.
Figures


Comment in
-
A treatment bridge for infants with hemophilia.Blood. 2024 Apr 4;143(14):1317-1318. doi: 10.1182/blood.2023023576. Blood. 2024. PMID: 38573607 No abstract available.
References
-
- Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(suppl 6):1–158. - PubMed
-
- Blanchette VS, Key NS, Ljung LR, et al. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12(11):1935–1939. - PubMed
-
- Zwagemaker A-F, Gouw SC, Jansen JS, et al. Incidence and mortality rates of intracranial hemorrhage in hemophilia: a systematic review and meta-analysis. Blood. 2021;138(26):2853–2873. - PubMed

