SARS-CoV-2 Rebound With and Without Use of COVID-19 Oral Antivirals
- PMID: 38127665
- PMCID: PMC10754268
- DOI: 10.15585/mmwr.mm7251a1
SARS-CoV-2 Rebound With and Without Use of COVID-19 Oral Antivirals
Abstract
Early treatment with a first-line therapy (nirmatrelvir/ritonavir [Paxlovid] or remdesivir) or second-line therapy (molnupiravir) prevents hospitalization and death among patients with mild-to-moderate COVID-19 who are at risk for severe disease and is recommended by the National Institutes of Health COVID-19 Treatment Guidelines. On May 25, 2023, the Food and Drug Administration approved nirmatrelvir/ritonavir for treatment of adults at high risk for severe disease. Although antiviral therapies are widely available, they are underutilized, possibly because of reports of SARS-CoV-2 rebound after treatment. To enhance current understanding of rebound, CDC reviewed SARS-CoV-2 rebound studies published during February 1, 2020- November 29, 2023. Overall, seven of 23 studies that met inclusion criteria, one randomized trial and six observational studies, compared rebound for persons who received antiviral treatment with that for persons who did not receive antiviral treatment. In four studies, including the randomized trial, no statistically significant difference in rebound rates was identified among persons receiving treatment and those not receiving treatment. Depending on the definition used, the prevalence of rebound varied. No hospitalizations or deaths were reported among outpatients who experienced rebound, because COVID-19 signs and symptoms were mild. Persons receiving antiviral treatment might be at higher risk for rebound compared with persons not receiving treatment because of host factors or treatment-induced viral suppression early in the course of illness. The potential for rebound should not deter clinicians from prescribing lifesaving antiviral treatments when indicated to prevent morbidity and mortality from COVID-19.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
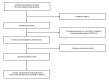
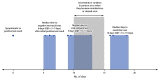
References
-
- National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. Washington, DC: US Department of Health and Human Services, National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/ Accessed December 5, 2023. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

