Influenza, Updated COVID-19, and Respiratory Syncytial Virus Vaccination Coverage Among Adults - United States, Fall 2023
- PMID: 38127675
- PMCID: PMC10754266
- DOI: 10.15585/mmwr.mm7251a4
Influenza, Updated COVID-19, and Respiratory Syncytial Virus Vaccination Coverage Among Adults - United States, Fall 2023
Abstract
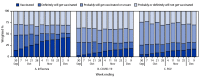
During the 2023-24 respiratory virus season, the Advisory Committee on Immunization Practices recommends influenza and COVID-19 vaccines for all persons aged ≥6 months, and respiratory syncytial virus (RSV) vaccine is recommended for persons aged ≥60 years (using shared clinical decision-making), and for pregnant persons. Data from the National Immunization Survey-Adult COVID Module, a random-digit-dialed cellular telephone survey of U.S. adults aged ≥18 years, are used to monitor influenza, COVID-19, and RSV vaccination coverage. By December 9, 2023, an estimated 42.2% and 18.3% of adults aged ≥18 years reported receiving an influenza and updated 2023-2024 COVID-19 vaccine, respectively; 17.0% of adults aged ≥60 years had received RSV vaccine. Coverage varied by demographic characteristics. Overall, approximately 27% and 41% of adults aged ≥18 years and 53% of adults aged ≥60 years reported that they definitely or probably will be vaccinated or were unsure whether they would be vaccinated against influenza, COVID-19, and RSV, respectively. Strong provider recommendations for and offers of vaccination could increase influenza, COVID-19, and RSV vaccination coverage. Immunization programs and vaccination partners are encouraged to use these data to understand vaccination patterns and attitudes toward vaccination in their jurisdictions to guide planning, implementation, strengthening, and evaluation of vaccination activities.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures

References
-
- Grohskopf LA, Blanton LH, Ferdinands JM, et al.. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2023–24 influenza season. MMWR Recomm Rep 2023;72(No. RR-1):1–25. 10.15585/mmwr.rr7202a1 - DOI - PMC - PubMed
-
- Regan JJ, Moulia DL, Link-Gelles R, et al. Use of updated COVID-19 vaccines 2023–2024 formula for persons aged ≥6 months: recommendations of the Advisory Committee on Immunization Practices—United States, September 2023. MMWR Morb Mortal Wkly Rep 2023;72:1140–6. 10.15585/mmwr.mm7242e1 - DOI - PMC - PubMed
-
- Black CL, O’Halloran A, Hung MC, et al.; Influenza-Associated Hospitalization Surveillance Network. Vital signs: influenza hospitalizations and vaccination coverage by race and ethnicity—United States, 2009–10 through 2021–22 influenza seasons. MMWR Morb Mortal Wkly Rep 2022;71:1366–73. 10.15585/mmwr.mm7143e1 - DOI - PMC - PubMed
-
- Copeland KR, Ganesh N, Liu L, Santibanez TA, Singleton JA. Statistical improvements in weekly-updated cumulative estimates of flu vaccination coverage for children in the United States. In: Proceedings of the Survey Research Methods Section, American Statistical Association, August 8–12, 2021. http://www.asasrms.org/Proceedings/y2021/files/1912282.pdf
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

