Long-Chain Polyunsaturated Fatty Acids Accelerate the Rate of Insulin Aggregation and Enhance Toxicity of Insulin Aggregates
- PMID: 38127718
- PMCID: PMC10862472
- DOI: 10.1021/acschemneuro.3c00583
Long-Chain Polyunsaturated Fatty Acids Accelerate the Rate of Insulin Aggregation and Enhance Toxicity of Insulin Aggregates
Abstract
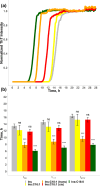
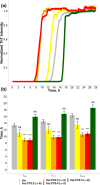
Long-chain polyunsaturated fatty acids (LCPUFAs) are essential components of a human diet. These molecules are critically important for cognitive attention and memory, mood states, coronary circulation, and cirrhosis. However, recently reported findings demonstrated that docosahexaenoic (DHA) and arachidonic acids (ARA), ω-3 and ω-6 LCPUFAs, accelerated the aggregation rates of insulin and α-synuclein, proteins that are directly linked to diabetes type 2 and Parkinson's disease, respectively. Furthermore, both DHA and ARA uniquely altered the structure and toxicity of the corresponding protein aggregates. Our objective is to ascertain whether other LCPUFAs, alongside long-chain unsaturated fatty acid (LCUFA) proteins, exhibit similar effects on amyloidogenic proteins. To explore this matter, we investigated the effect of 10 different LCPUFAs and LCUFAs on the rate of insulin aggregation. We found that all of the analyzed fatty acids strongly accelerated insulin aggregation. Moreover, we found that protein aggregates that were formed in the presence of these fatty acids exerted significantly higher cell toxicity compared with insulin fibrils grown in the lipid-free environment. These findings show that interactions between amyloid-associated proteins and LCPUFAs can be the underlying molecular cause of neurodegenerative diseases.
Keywords: LCPUFAs; LCUFAs; fibrils; insulin; oligomers; toxicity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Unsaturated fatty acids uniquely alter aggregation rate of α-synuclein and insulin and change the secondary structure and toxicity of amyloid aggregates formed in their presence.FASEB J. 2023 Jul;37(7):e22972. doi: 10.1096/fj.202300003R. FASEB J. 2023. PMID: 37302013 Free PMC article.
-
Elucidating the Role of Lipids in the Aggregation of Amyloidogenic Proteins.Acc Chem Res. 2023 Nov 7;56(21):2898-2906. doi: 10.1021/acs.accounts.3c00386. Epub 2023 Oct 12. Acc Chem Res. 2023. PMID: 37824095 Free PMC article.
-
Fatty Acids Reverse the Supramolecular Chirality of Insulin Fibrils.J Phys Chem Lett. 2023 Aug 3;14(30):6935-6939. doi: 10.1021/acs.jpclett.3c01527. Epub 2023 Jul 27. J Phys Chem Lett. 2023. PMID: 37498215 Free PMC article.
-
Transport mechanisms for long-chain polyunsaturated fatty acids in the human placenta.Am J Clin Nutr. 2000 Jan;71(1 Suppl):315S-22S. doi: 10.1093/ajcn/71.1.315s. Am J Clin Nutr. 2000. PMID: 10617989 Review.
-
Long-chain polyunsaturated fatty acids interact with nitric oxide, superoxide anion, and transforming growth factor-beta to prevent human essential hypertension.Eur J Clin Nutr. 2004 Feb;58(2):195-203. doi: 10.1038/sj.ejcn.1601766. Eur J Clin Nutr. 2004. PMID: 14749737 Review.
Cited by
-
Cholesterol Accelerates Aggregation of α-Synuclein Simultaneously Increasing the Toxicity of Amyloid Fibrils.ACS Chem Neurosci. 2024 Nov 6;15(21):4075-4081. doi: 10.1021/acschemneuro.4c00501. Epub 2024 Oct 29. ACS Chem Neurosci. 2024. PMID: 39469734 Free PMC article.
-
Lipids determine the toxicity of human islet polypeptide aggregates in vivo.J Biol Chem. 2025 Jan;301(1):108029. doi: 10.1016/j.jbc.2024.108029. Epub 2024 Nov 29. J Biol Chem. 2025. PMID: 39615682 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

