Fast cycling culture of the annelid model Platynereis dumerilii
- PMID: 38127889
- PMCID: PMC10735030
- DOI: 10.1371/journal.pone.0295290
Fast cycling culture of the annelid model Platynereis dumerilii
Abstract
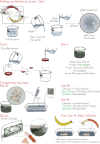
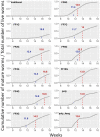
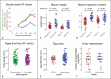
Platynereis dumerilii, a marine annelid, is a model animal that has gained popularity in various fields such as developmental biology, biological rhythms, nervous system organization and physiology, behaviour, reproductive biology, and epigenetic regulation. The transparency of P. dumerilii tissues at all developmental stages makes it easy to perform live microscopic imaging of all cell types. In addition, the slow-evolving genome of P. dumerilii and its phylogenetic position as a representative of the vast branch of Lophotrochozoans add to its evolutionary significance. Although P. dumerilii is amenable to transgenesis and CRISPR-Cas9 knockouts, its relatively long and indefinite life cycle, as well as its semelparous reproduction have been hindrances to its adoption as a reverse genetics model. To overcome this limitation, an adapted culturing method has been developed allowing much faster life cycling, with median reproductive age at 13-14 weeks instead of 25-35 weeks using the traditional protocol. A low worm density in boxes and a strictly controlled feeding regime are important factors for the rapid growth and health of the worms. This culture method has several advantages, such as being much more compact, not requiring air bubbling or an artificial moonlight regime for synchronized sexual maturation and necessitating only limited water change. A full protocol for worm care and handling is provided.
Copyright: © 2023 Legras et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
A scalable culturing system for the marine annelid Platynereis dumerilii.PLoS One. 2019 Dec 5;14(12):e0226156. doi: 10.1371/journal.pone.0226156. eCollection 2019. PLoS One. 2019. PMID: 31805142 Free PMC article.
-
PdumBase: a transcriptome database and research tool for Platynereis dumerilii and early development of other metazoans.BMC Genomics. 2018 Aug 16;19(1):618. doi: 10.1186/s12864-018-4987-0. BMC Genomics. 2018. PMID: 30115014 Free PMC article.
-
Segment number threshold determines juvenile onset of germline cluster expansion in Platynereis dumerilii.J Exp Zool B Mol Dev Evol. 2022 Jun;338(4):225-240. doi: 10.1002/jez.b.23100. Epub 2021 Nov 18. J Exp Zool B Mol Dev Evol. 2022. PMID: 34793615 Free PMC article.
-
Genetic and genomic tools for the marine annelid Platynereis dumerilii.Genetics. 2014 May;197(1):19-31. doi: 10.1534/genetics.112.148254. Genetics. 2014. PMID: 24807110 Free PMC article. Review.
-
Towards a systems-level understanding of development in the marine annelid Platynereis dumerilii.Curr Opin Genet Dev. 2016 Aug;39:175-181. doi: 10.1016/j.gde.2016.07.005. Epub 2016 Aug 6. Curr Opin Genet Dev. 2016. PMID: 27501412 Review.
Cited by
-
The cost and payout of age on germline regeneration and sexual maturation in Platynereis dumerilii.bioRxiv [Preprint]. 2024 Jan 22:2024.01.22.576726. doi: 10.1101/2024.01.22.576726. bioRxiv. 2024. Update in: Dev Biol. 2024 Sep;513:33-49. doi: 10.1016/j.ydbio.2024.05.013. PMID: 38328233 Free PMC article. Updated. Preprint.
-
Developmental stage dependent effects of posterior and germline regeneration on sexual maturation in Platynereis dumerilii.Dev Biol. 2024 Sep;513:33-49. doi: 10.1016/j.ydbio.2024.05.013. Epub 2024 May 24. Dev Biol. 2024. PMID: 38797257 Free PMC article.
-
Microalgal biofilm induces larval settlement in the model marine worm Platynereis dumerilii.R Soc Open Sci. 2024 Sep 18;11(9):240274. doi: 10.1098/rsos.240274. eCollection 2024 Sep. R Soc Open Sci. 2024. PMID: 39295916 Free PMC article.
References
-
- Ankeny R, Leonelli S. Model Organisms. Model Organisms. Cambridge University Press; 2020.
MeSH terms
LinkOut - more resources
Full Text Sources

