FLAgellum Member 8 modulates extravascular distribution of African trypanosomes
- PMID: 38127941
- PMCID: PMC10769064
- DOI: 10.1371/journal.ppat.1011220
FLAgellum Member 8 modulates extravascular distribution of African trypanosomes
Abstract
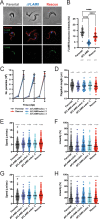
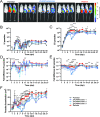
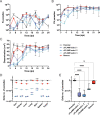
In the mammalian host, the biology of tissue-dwelling Trypanosoma brucei parasites is not completely understood, especially the mechanisms involved in their extravascular colonization. The trypanosome flagellum is an essential organelle in multiple aspects of the parasites' development. The flagellar protein termed FLAgellar Member 8 (FLAM8) acts as a docking platform for a pool of cyclic AMP response protein 3 (CARP3) that is involved in signaling. FLAM8 exhibits a stage-specific distribution suggesting specific functions in the mammalian and vector stages of the parasite. Analyses of knockdown and knockout trypanosomes in their mammalian forms demonstrated that FLAM8 is not essential in vitro for survival, growth, motility and stumpy differentiation. Functional investigations in experimental infections showed that FLAM8-deprived trypanosomes can establish and maintain an infection in the blood circulation and differentiate into insect transmissible forms. However, quantitative bioluminescence imaging and gene expression analysis revealed that FLAM8-null parasites exhibit a significantly impaired dissemination in the extravascular compartment, that is restored by the addition of a single rescue copy of FLAM8. In vitro trans-endothelial migration assays revealed significant defects in trypanosomes lacking FLAM8. FLAM8 is the first flagellar component shown to modulate T. brucei distribution in the host tissues, possibly through sensing functions, contributing to the maintenance of extravascular parasite populations in mammalian anatomical niches, especially in the skin.
Copyright: © 2023 Calvo-Alvarez et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures