Targeted solutions to increase dolutegravir coverage, viral load testing coverage, and viral suppression among children living with HIV in Togo: An analysis of routine facility data
- PMID: 38128036
- PMCID: PMC10735014
- DOI: 10.1371/journal.pone.0296293
Targeted solutions to increase dolutegravir coverage, viral load testing coverage, and viral suppression among children living with HIV in Togo: An analysis of routine facility data
Abstract
Background: According to UNAIDS, Togo halved AIDS-related deaths among children ages 0-14 from 2010 to 2020. However, available data show low dolutegravir (DTG)-containing antiretroviral therapy (ART) coverage and low viral load suppression (VLS) among children living with HIV (CLHIV). We analyzed routine facility data before and after implementation of root-cause-based solutions for improving DTG coverage, viral load (VL) testing coverage, and VLS among CLHIV.
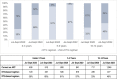
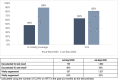
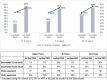
Description: We analyzed routine data for CLHIV ≤14 years from October 2019 through September 2022. We assessed proportion of CLHIV on ART receiving DTG, VL testing coverage (CLHIV on ART with documented VL test result), and VLS (CLHIV with documented VL test result of <1,000 copies among those with test result). From October 2019 to September 2020, 52% were on a DTG-containing regimen, 48% had documented VL test results, and 64% had VLS. Site-level teams conducted a root-cause analysis and designed corresponding solutions implemented beginning October 2020: line listing and contacting eligible CLHIV to start/transition to DTG-containing regimen and collect VL samples; ART adherence support; monthly DTG stock monitoring; tracking pending VL test results through laboratory focal persons; documenting VL test results; and informing caregivers within one week if CLHIV not virally suppressed. Granular data were used to prioritize technical assistance to sites with lowest DTG coverage, VL testing coverage, and VLS.
Results: From baseline (October 2019-September 2020) to endline (October 2021-September 2022), increases were observed for DTG coverage (52% to 71%), VL testing coverage (48% to 90%), and VLS (64% to 82%). Age-disaggregated data showed positive trends.
Conclusions: Root-cause-based solutions and granular data use increased DTG coverage, resulting in increased VL testing and VLS among CLHIV. These interventions should be scaled and become the national standard of care.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS data 2022. Geneva: UNAIDS; 2022. Available from: https://www.unaids.org/en/resources/documents/2023/2022_unaids_data. - PubMed
-
- Viani RM, Ruel T, Alvero C, Fenton T, Acosta EP, Hazra R, et al. Long-term safety and efficacy of dolutegravir in treatment-experienced adolescents with human immunodeficiency virus infection: results of the IMPAACT P1093 study. J Pediatric Infect Dis Soc. 2020;9(2):159–65. doi: 10.1093/jpids/piy139 - DOI - PMC - PubMed
-
- World Health Organization (WHO). Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV. Geneva: WHO; 2018. Available from: https://www.who.int/publications/i/item/WHO-CDS-HIV-18.51.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical