Mitochondrial fatty acid synthesis is an emergent central regulator of mammalian oxidative metabolism
- PMID: 38128528
- PMCID: PMC10843818
- DOI: 10.1016/j.cmet.2023.11.017
Mitochondrial fatty acid synthesis is an emergent central regulator of mammalian oxidative metabolism
Abstract
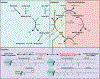
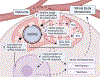
Contrary to their well-known functions in nutrient breakdown, mitochondria are also important biosynthetic hubs and express an evolutionarily conserved mitochondrial fatty acid synthesis (mtFAS) pathway. mtFAS builds lipoic acid and longer saturated fatty acids, but its exact products, their ultimate destination in cells, and the cellular significance of the pathway are all active research questions. Moreover, why mitochondria need mtFAS despite their well-defined ability to import fatty acids is still unclear. The identification of patients with inborn errors of metabolism in mtFAS genes has sparked fresh research interest in the pathway. New mammalian models have provided insights into how mtFAS coordinates many aspects of oxidative mitochondrial metabolism and raise questions about its role in diseases such as obesity, diabetes, and heart failure. In this review, we discuss the products of mtFAS, their function, and the consequences of mtFAS impairment across models and in metabolic disease.
Keywords: fatty acids; inborn errors of metabolism; lipid metabolism; lipids; mitochondria; mitochondrial fatty acid synthesis; mouse models; mtFAS.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Mikolajczyk S, and Brody S (1990). De novo fatty acid synthesis mediated by acyl-carrier protein in Neurospora crassa mitochondria. Eur. J. Biochem 187, 431–437. - PubMed
-
- Zensen R, Husmann H, Schneider R, Peine T, and Weiss H (1992). De novo synthesis and desaturation of fatty acids at the mitochondrial acyl-carrier protein, a subunit of NADH:ubiquinone oxidoreductase in Neurospora crassa. FEBS Lett. 310, 179–181. - PubMed
-
- Brody S, Oh C, Hoja U, and Schweizer E (1997). Mitochondrial acyl carrier protein is involved in lipoic acid synthesis in Saccharomyces cerevisiae. FEBS Lett. 408, 217–220. - PubMed
-
- Jordan SW, and Cronan JE Jr, (1997). A new metabolic link. The acyl carrier protein of lipid synthesis donates lipoic acid to the pyruvate dehydrogenase complex in Escherichia coli and mitochondria. J. Biol. Chem 272, 17903–17906. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

