Disrupted RNA editing in beta cells mimics early-stage type 1 diabetes
- PMID: 38128529
- PMCID: PMC10843671
- DOI: 10.1016/j.cmet.2023.11.011
Disrupted RNA editing in beta cells mimics early-stage type 1 diabetes
Abstract
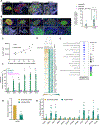
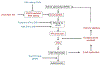
A major hypothesis for the etiology of type 1 diabetes (T1D) postulates initiation by viral infection, leading to double-stranded RNA (dsRNA)-mediated interferon response and inflammation; however, a causal virus has not been identified. Here, we use a mouse model, corroborated with human islet data, to demonstrate that endogenous dsRNA in beta cells can lead to a diabetogenic immune response, thus identifying a virus-independent mechanism for T1D initiation. We found that disruption of the RNA editing enzyme adenosine deaminases acting on RNA (ADAR) in beta cells triggers a massive interferon response, islet inflammation, and beta cell failure and destruction, with features bearing striking similarity to early-stage human T1D. Glycolysis via calcium enhances the interferon response, suggesting an actionable vicious cycle of inflammation and increased beta cell workload.
Keywords: RNA editing; beta cells; interferon response; islet inflammation; metabolic stress; type 1 diabetes.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

