Loss-of-function cancer-linked mutations in the EIF4G2 non-canonical translation initiation factor
- PMID: 38129098
- PMCID: PMC10746786
- DOI: 10.26508/lsa.202302338
Loss-of-function cancer-linked mutations in the EIF4G2 non-canonical translation initiation factor
Abstract
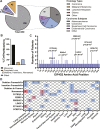
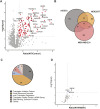
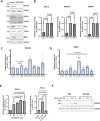
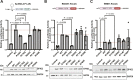
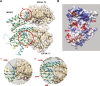
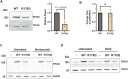
Tumor cells often exploit the protein translation machinery, resulting in enhanced protein expression essential for tumor growth. Since canonical translation initiation is often suppressed because of cell stress in the tumor microenvironment, non-canonical translation initiation mechanisms become particularly important for shaping the tumor proteome. EIF4G2 is a non-canonical translation initiation factor that mediates internal ribosome entry site (IRES)- and uORF-dependent initiation mechanisms, which can be used to modulate protein expression in cancer. Here, we explored the contribution of EIF4G2 to cancer by screening the COSMIC database for EIF4G2 somatic mutations in cancer patients. Functional examination of missense mutations revealed deleterious effects on EIF4G2 protein-protein interactions and, importantly, on its ability to mediate non-canonical translation initiation. Specifically, one mutation, R178Q, led to reductions in protein expression and near-complete loss of function. Two other mutations within the MIF4G domain specifically affected EIF4G2's ability to mediate IRES-dependent translation initiation but not that of target mRNAs with uORFs. These results shed light on both the structure-function of EIF4G2 and its potential tumor suppressor effects.
© 2023 Meril et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
