ANKS1B encoded AIDA-1 regulates social behaviors by controlling oligodendrocyte function
- PMID: 38129387
- PMCID: PMC10739966
- DOI: 10.1038/s41467-023-43438-1
ANKS1B encoded AIDA-1 regulates social behaviors by controlling oligodendrocyte function
Abstract
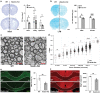
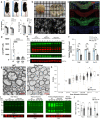
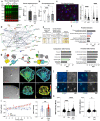
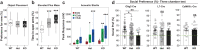
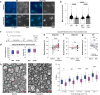
Heterozygous deletions in the ANKS1B gene cause ANKS1B neurodevelopmental syndrome (ANDS), a rare genetic disease characterized by autism spectrum disorder (ASD), attention deficit/hyperactivity disorder, and speech and motor deficits. The ANKS1B gene encodes for AIDA-1, a protein that is enriched at neuronal synapses and regulates synaptic plasticity. Here we report an unexpected role for oligodendroglial deficits in ANDS pathophysiology. We show that Anks1b-deficient mouse models display deficits in oligodendrocyte maturation, myelination, and Rac1 function, and recapitulate white matter abnormalities observed in ANDS patients. Selective loss of Anks1b from the oligodendrocyte lineage, but not from neuronal populations, leads to deficits in social preference and sensory reactivity previously observed in a brain-wide Anks1b haploinsufficiency model. Furthermore, we find that clemastine, an antihistamine shown to increase oligodendrocyte precursor cell maturation and central nervous system myelination, rescues deficits in social preference in 7-month-old Anks1b-deficient mice. Our work shows that deficits in social behaviors present in ANDS may originate from abnormal Rac1 activity within oligodendrocytes.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

