Central neuropathic pain
- PMID: 38129427
- PMCID: PMC11329872
- DOI: 10.1038/s41572-023-00484-9
Central neuropathic pain
Abstract
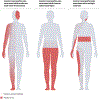
Central neuropathic pain arises from a lesion or disease of the central somatosensory nervous system such as brain injury, spinal cord injury, stroke, multiple sclerosis or related neuroinflammatory conditions. The incidence of central neuropathic pain differs based on its underlying cause. Individuals with spinal cord injury are at the highest risk; however, central post-stroke pain is the most prevalent form of central neuropathic pain worldwide. The mechanisms that underlie central neuropathic pain are not fully understood, but the pathophysiology likely involves intricate interactions and maladaptive plasticity within spinal circuits and brain circuits associated with nociception and antinociception coupled with neuronal hyperexcitability. Modulation of neuronal activity, neuron-glia and neuro-immune interactions and targeting pain-related alterations in brain connectivity, represent potential therapeutic approaches. Current evidence-based pharmacological treatments include antidepressants and gabapentinoids as first-line options. Non-pharmacological pain management options include self-management strategies, exercise and neuromodulation. A comprehensive pain history and clinical examination form the foundation of central neuropathic pain classification, identification of potential risk factors and stratification of patients for clinical trials. Advanced neurophysiological and neuroimaging techniques hold promise to improve the understanding of mechanisms that underlie central neuropathic pain and as predictive biomarkers of treatment outcome.
© 2023. Springer Nature Limited.
Figures


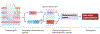



References
-
- Jensen TS et al. A new definition of neuropathic pain. Pain 152, 2204–2205 (2011). - PubMed
-
-
Scholz J. et al. The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain 160, 53–59 (2019).
This paper is an overview of conditions included in the International Classification of Diseases 11th Revision classification of chronic neuropathic pain, including CNP.
-
-
- Widerström-Noga E, Loeser JD, Jensen TS & Finnerup NB AAPT diagnostic criteria for central neuropathic pain. J. Pain 18, 1417–1426 (2017). - PubMed
-
- Widerström-Noga E, Felix ER, Adcock JP, Escalona M & Tibbett J Multidimensional neuropathic pain phenotypes after spinal cord injury. J. Neurotrauma 33, 482–492 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

