Inhibition of Drp1 orchestrates the responsiveness of breast cancer cells to paclitaxel but insignificantly relieves paclitaxel-related ovarian damage in mice
- PMID: 38129495
- PMCID: PMC10739747
- DOI: 10.1038/s41598-023-49578-0
Inhibition of Drp1 orchestrates the responsiveness of breast cancer cells to paclitaxel but insignificantly relieves paclitaxel-related ovarian damage in mice
Erratum in
-
Author Correction: Inhibition of Drp1 orchestrates the responsiveness of breast cancer cells to paclitaxel but insignificantly relieves paclitaxel‑related ovarian damage in mice.Sci Rep. 2024 Apr 29;14(1):9788. doi: 10.1038/s41598-024-60556-y. Sci Rep. 2024. PMID: 38684881 Free PMC article. No abstract available.
Abstract
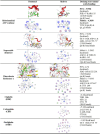
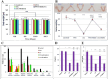
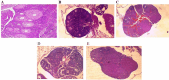
Chemoresistance and chemotherapy-related ovarian damage are well-reported in breast cancer (BC) young patients. Herein, the inhibition of the mitochondrial fission was invested to explore its chemosensitizing role in Paclitaxel (PTX)-resistant cells, and its ability to restore the ovarian integrity in mice receiving PTX or cisplatin chemotherapy. To establish these aims, PTX-resistance was generated in BC cells, which were treated with PTX in combination with Drp1 deficiency, via mdivi-1, or Drp1-specific siRNA transfection. Furthermore, the alterations in the ovarian structure and the endocrine-related hormones were explored in mice receiving repetitive doses of PTX or cisplatin. We found that combining PTX with mdivi-1 improved cell responsiveness to PTX, induced apoptosis- and autophagy-mediated cell death, and relieved cellular oxidative stress. Additionally, the expression of PCNA1 and cyclin B1 genes were downregulated, meanwhile, p53, p21, and mitochondrial fusion proteins (Mfu1&Mfu2) were increased. The in vivo investigations in mice demonstrated that PTX induced gonadotoxic damage similar to cisplatin, whereas dual treatment of mice with PTX+ mdivi-1 failed to restore their normal follicular count and the circulating levels of E2 and AMH hormones. These results suggested that combining Drp1 inhibition with PTX resensitized breast cancer cells to PTX but failed to offer enough protection against chemotherapy-related gonadotoxicity.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

