The Cousa objective: a long-working distance air objective for multiphoton imaging in vivo
- PMID: 38129618
- PMCID: PMC10776402
- DOI: 10.1038/s41592-023-02098-1
The Cousa objective: a long-working distance air objective for multiphoton imaging in vivo
Abstract
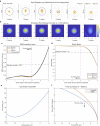
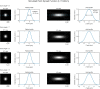
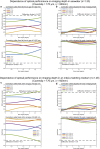
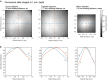
Multiphoton microscopy can resolve fluorescent structures and dynamics deep in scattering tissue and has transformed neural imaging, but applying this technique in vivo can be limited by the mechanical and optical constraints of conventional objectives. Short working distance objectives can collide with compact surgical windows or other instrumentation and preclude imaging. Here we present an ultra-long working distance (20 mm) air objective called the Cousa objective. It is optimized for performance across multiphoton imaging wavelengths, offers a more than 4 mm2 field of view with submicrometer lateral resolution and is compatible with commonly used multiphoton imaging systems. A novel mechanical design, wider than typical microscope objectives, enabled this combination of specifications. We share the full optical prescription, and report performance including in vivo two-photon and three-photon imaging in an array of species and preparations, including nonhuman primates. The Cousa objective can enable a range of experiments in neuroscience and beyond.
© 2023. The Author(s).
Conflict of interest statement
The design of the objective is not patented, and it will not be patented in the future. All designs originating in this report are free for reuse, no licensing or material transfer agreements are required. Notification is not required either, but only humbly requested. S.L.S. is a paid consultant for companies that sell optics and multiphoton microscopes. C.-H.Y. and S.L.S. have interests in the company Pacific Optica. The other authors declare no competing interests.
Figures











References
MeSH terms
Substances
Grants and funding
- P30 EY026878/EY/NEI NIH HHS/United States
- R01 NS091335/NS/NINDS NIH HHS/United States
- R01 DC003896/DC/NIDCD NIH HHS/United States
- T32 NS091018/NS/NINDS NIH HHS/United States
- R01 NS121919/NS/NINDS NIH HHS/United States
- R01 EY011488/EY/NEI NIH HHS/United States
- U01 NS115530/NS/NINDS NIH HHS/United States
- R01 NS128079/NS/NINDS NIH HHS/United States
- R01 EY032416/EY/NEI NIH HHS/United States
- F32 EY031133/EY/NEI NIH HHS/United States
- T32 OD011089/OD/NIH HHS/United States
- R01 MH099045/MH/NIMH NIH HHS/United States
- U01 NS115585/NS/NINDS NIH HHS/United States
- R21 DC020325/DC/NIDCD NIH HHS/United States
- R01 EY030893/EY/NEI NIH HHS/United States
- U01 NS103488/NS/NINDS NIH HHS/United States
- R01 EY024294/EY/NEI NIH HHS/United States
- P30 EY026877/EY/NEI NIH HHS/United States
- R01 EY006821/EY/NEI NIH HHS/United States
- DP1 EY033975/EY/NEI NIH HHS/United States
- R01 EY022951/EY/NEI NIH HHS/United States
- R01 EY035378/EY/NEI NIH HHS/United States
- U01 NS126057/NS/NINDS NIH HHS/United States

