Hyperglycemia-induced STING signaling activation leads to aortic endothelial injury in diabetes
- PMID: 38129863
- PMCID: PMC10734150
- DOI: 10.1186/s12964-023-01393-w
Hyperglycemia-induced STING signaling activation leads to aortic endothelial injury in diabetes
Abstract
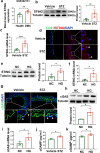
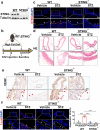
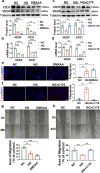
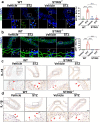
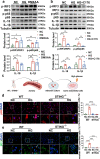
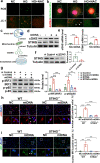
Hyperglycaemia-induced endothelial dysfunction is a key factor in the pathogenesis of diabetic microangiopathy and macroangiopathy. STING, which is a newly discovered regulator of innate immunity, has also been reported to play an important role in various metabolic diseases. However, the role of STING in diabetes-induced endothelial cell dysfunction is unknown. In this study, we established a diabetic macroangiopathy mouse model by streptozotocin (STZ) injection combined with high-fat diet (HFD) feeding and a glucotoxicity cell model in high glucose (HG)-treated rat aortic endothelial cells (RAECs). We found that STING expression was specifically increased in the endothelial cells of diabetic arteries, as well as in HG-treated RAECs. Moreover, genetic deletion of STING significantly ameliorated diabetes-induced endothelial cell dysfunction and apoptosis in vivo. Likewise, STING inhibition by C-176 reversed HG-induced migration dysfunction and apoptosis in RAECs, whereas STING activation by DMXAA resulted in migration dysfunction and apoptosis. Mechanistically, hyperglycaemia-induced oxidative stress promoted endothelial mitochondrial dysfunction and mtDNA release, which subsequently activated the cGAS-STING system and the cGAS-STING-dependent IRF3/NF-kB pathway, ultimately resulting in inflammation and apoptosis. In conclusion, our study identified a novel role of STING in diabetes-induced aortic endothelial cell injury and suggested that STING inhibition was a potential new therapeutic strategy for the treatment of diabetic macroangiopathy. Video Abstract.
Keywords: Aortic endothelial cells; Diabetes; Hyperglycemia; STING.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. Idf diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

