Succinate-induced macrophage polarization and RBP4 secretion promote vascular sprouting in ocular neovascularization
- PMID: 38129891
- PMCID: PMC10734053
- DOI: 10.1186/s12974-023-02998-1
Succinate-induced macrophage polarization and RBP4 secretion promote vascular sprouting in ocular neovascularization
Abstract
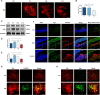
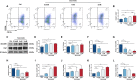
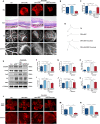
Pathological neovascularization is a pivotal biological process in wet age-related macular degeneration (AMD), retinopathy of prematurity (ROP) and proliferative diabetic retinopathy (PDR), in which macrophages (Mφs) play a key role. Tip cell specialization is critical in angiogenesis; however, its interconnection with the surrounding immune environment remains unclear. Succinate is an intermediate in the tricarboxylic acid (TCA) cycle and was significantly elevated in patients with wet AMD by metabolomics. Advanced experiments revealed that SUCNR1 expression in Mφ and M2 polarization was detected in abnormal vessels of choroidal neovascularization (CNV) and oxygen-induced retinopathy (OIR) models. Succinate-induced M2 polarization via SUCNR1, which facilitated vascular endothelial cell (EC) migration, invasion, and tubulation, thus promoting angiogenesis in pathological neovascularization. Furthermore, evidence indicated that succinate triggered the release of RBP4 from Mφs into the surroundings to regulate endothelial sprouting and pathological angiogenesis via VEGFR2, a marker of tip cell formation. In conclusion, our results suggest that succinate represents a novel class of vasculature-inducing factors that modulate Mφ polarization and the RBP4/VEGFR2 pathway to induce pathological angiogenic signaling through tip cell specialization.
Keywords: Choroidal neovascularization; Macrophage; Oxygen-induced retinopathy; Succinate; Tip cells.
© 2023. The Author(s).
Conflict of interest statement
The authors declared no competing or financial interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

