Lactobacillus Reuteri 6475 Prevents Bone Loss in a Clinically Relevant Oral Model of Glucocorticoid-Induced Osteoporosis in Male CD-1 Mice
- PMID: 38130770
- PMCID: PMC10731127
- DOI: 10.1002/jbm4.10805
Lactobacillus Reuteri 6475 Prevents Bone Loss in a Clinically Relevant Oral Model of Glucocorticoid-Induced Osteoporosis in Male CD-1 Mice
Abstract
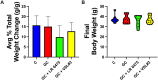
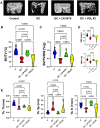
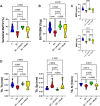
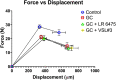
Glucocorticoids (GCs) are commonly used anti-inflammatory medications with significant side effects, including glucocorticoid-induced osteoporosis (GIO). We have previously demonstrated that chronic subcutaneous GC treatment in mice leads to gut barrier dysfunction and trabecular bone loss. We further showed that treating with probiotics or barrier enhancers improves gut barrier function and prevents GIO. The overall goal of this study was to test if probiotics could prevent GC-induced gut barrier dysfunction and bone loss in a clinically relevant oral-GC model of GIO. Eight-week-old male CD-1 mice were treated with vehicle or corticosterone in the drinking water for 4 weeks and administered probiotics Lactobacillus reuteri ATCC 6475 (LR 6475) or VSL#3 thrice weekly via oral gavage. As expected, GC treatment led to significant gut barrier dysfunction (assessed by measuring serum endotoxin levels) and bone loss after 4 weeks. Serum endotoxin levels significantly and negatively correlated with bone volume. Importantly, LR 6475 treatment effectively prevented both GC-induced increase in serum endotoxin and trabecular bone loss. VSL#3 had intermediate results, not differing from either control or GC-treated animals. GC-induced reductions in femur length, cortical thickness, and cortical area were not affected by probiotic treatment. Taken together, these results are the first to demonstrate that LR 6475 effectively prevents the detrimental effects of GC treatment on gut barrier, which correlates with enhanced trabecular bone health in an oral mouse model of GIO. © 2023 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
Keywords: BARRIER; GLUCOCORTICOID; GUT‐BONE INTERACTIONS; OSTEOPOROSIS; PROBIOTIC.
© 2023 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
Figures

Similar articles
-
Glucocorticoid-induced osteoporosis is prevented by dietary prune in female mice.Front Cell Dev Biol. 2024 Feb 5;11:1324649. doi: 10.3389/fcell.2023.1324649. eCollection 2023. Front Cell Dev Biol. 2024. PMID: 38375074 Free PMC article.
-
Korean red ginseng extract prevents bone loss in an oral model of glucocorticoid induced osteoporosis in mice.Front Pharmacol. 2024 Mar 12;15:1268134. doi: 10.3389/fphar.2024.1268134. eCollection 2024. Front Pharmacol. 2024. PMID: 38533264 Free PMC article.
-
Involvement of the Gut Microbiota and Barrier Function in Glucocorticoid-Induced Osteoporosis.J Bone Miner Res. 2020 Apr;35(4):801-820. doi: 10.1002/jbmr.3947. Epub 2020 Jan 23. J Bone Miner Res. 2020. PMID: 31886921
-
Advanced imaging assessment of bone fragility in glucocorticoid-induced osteoporosis.Bone. 2011 Jun 1;48(6):1221-31. doi: 10.1016/j.bone.2011.02.005. Epub 2011 Feb 20. Bone. 2011. PMID: 21320651 Review.
-
Secondary osteoporosis: underlying disease and the risk for glucocorticoid-induced osteoporosis.Clin Ther. 2004 Jan;26(1):1-14. doi: 10.1016/s0149-2918(04)90001-x. Clin Ther. 2004. PMID: 14996513 Review.
Cited by
-
Progress in Probiotic Science: Prospects of Functional Probiotic-Based Foods and Beverages.Int J Food Sci. 2025 Apr 14;2025:5567567. doi: 10.1155/ijfo/5567567. eCollection 2025. Int J Food Sci. 2025. PMID: 40259922 Free PMC article. Review.
-
Glucocorticoid-induced osteoporosis is prevented by dietary prune in female mice.Front Cell Dev Biol. 2024 Feb 5;11:1324649. doi: 10.3389/fcell.2023.1324649. eCollection 2023. Front Cell Dev Biol. 2024. PMID: 38375074 Free PMC article.
-
Korean red ginseng extract prevents bone loss in an oral model of glucocorticoid induced osteoporosis in mice.Front Pharmacol. 2024 Mar 12;15:1268134. doi: 10.3389/fphar.2024.1268134. eCollection 2024. Front Pharmacol. 2024. PMID: 38533264 Free PMC article.
-
The Improvement and Related Mechanism of Microecologics on the Sports Performance and Post-Exercise Recovery of Athletes: A Narrative Review.Nutrients. 2024 May 24;16(11):1602. doi: 10.3390/nu16111602. Nutrients. 2024. PMID: 38892536 Free PMC article. Review.
-
Cross-talks between osteoporosis and gut microbiome.World J Orthop. 2025 Mar 18;16(3):102274. doi: 10.5312/wjo.v16.i3.102274. eCollection 2025 Mar 18. World J Orthop. 2025. PMID: 40124724 Free PMC article. Review.
References
-
- Overman RA, Yeh J, Deal CL. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthrit Care Res. 2013;65(2):294–298. - PubMed
-
- Seibel MJ, Cooper MS, Zhou H. Glucocorticoid‐induced osteoporosis: mechanisms, management, and future perspectives. Lancet Diabetes Endocrinol. 2013;1(1):59–70. - PubMed
-
- Adami G, Saag KG. Glucocorticoid‐induced osteoporosis update. Curr Opin Rheumatol. 2019;31(4):388–393. - PubMed
-
- Adami G, Saag KG. Glucocorticoid‐induced osteoporosis: 2019 concise clinical review. Osteoporosis Int. 2019;30(6):1145–1156. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
