Idebenone Treatment in Patients with OPA1-Dominant Optic Atrophy: A Prospective Phase 2 Trial
- PMID: 38130806
- PMCID: PMC10732653
- DOI: 10.1080/01658107.2023.2251575
Idebenone Treatment in Patients with OPA1-Dominant Optic Atrophy: A Prospective Phase 2 Trial
Abstract
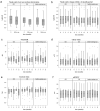
The aim of this study was to evaluate the therapeutic effect of idebenone in patients with OPA1-dominant optic atrophy (DOA). Sixteen patients with genetically confirmed OPA1-DOA were treated with 900 mg idebenone daily for 12 months. The primary endpoint was the best recovery/least deterioration of visual acuity. Secondary endpoints were the changes of visual acuity, colour vision, contrast sensitivity, visual field, peripapillary retinal nerve fibre layer thickness (pRNFLT), and visual-related quality of life. For the primary endpoint, a significant increase was observed for the right eye (p = .0027), for the left eye (p = .0111) and for the better-seeing eye (p = .0152). For visual fields, a significant improvement was observed for the left eye between baseline and 9 months (p = .0038). Regarding pRNFLT, a significant decrease was found for the left eye between baseline and 3 months (p = .0413) and between baseline and 6 months (p = .0448). In the visual function questionnaire, a significant improvement was observed in the subscale general vision (p = .0156) and in the composite score (p = .0256). In conclusion, best recovery of visual acuity improved, even though the amount of improvement was small. Furthermore, a maintenance of visual function after 12 months of idebenone intake could be observed as well as a significant improvement in vision-related quality of life.Whether this effect is due to idebenone treatment, the placebo effect, or is explainable by the natural progression of DOA, remains unclear. Trial registration: EU Clinical Trials Register, EudraCT Number: 2019-001493-28.
Keywords: Dominant optic atrophy; OPA1; idebenone; optic neuropathy; visual function.
© 2023 Taylor & Francis Group, LLC.
Conflict of interest statement
K. Valentin received travel reimbursements from Chiesi Pharmaceuticals GmbH, H. Aminfar received travel reimbursements from Santhera Pharmaceuticals and Chiesi Pharmaceuticals GmbH. T. Klopstock received travel reimbursements and speaker honoraria from Santhera Pharmaceuticals and Chiesi Pharmaceuticals GmbH and M. Schneider received speaker honoraria from Santhera Pharmaceuticals. T. Georgi, R. Riedl, C. Singer, and A. Wedrich report no competing interests.
Figures
References
LinkOut - more resources
Full Text Sources
