Analysis of treatment sequence and outcomes in patients with relapsed malignant peripheral nerve sheath tumors
- PMID: 38130899
- PMCID: PMC10733661
- DOI: 10.1093/noajnl/vdad156
Analysis of treatment sequence and outcomes in patients with relapsed malignant peripheral nerve sheath tumors
Abstract
Background: Malignant peripheral nerve sheath tumors (MPNST) are aggressive soft tissue sarcomas originating from cellular components within the nerve sheath. The incidence of MPNST is highest in people with neurofibromatosis type 1 (NF1), and MPNST is the leading cause of death for these individuals. Complete surgical resection is the only curative therapeutic option, but is often unfeasible due to tumor location, size, or presence of metastases. Evidence-based choices of chemotherapy for recurrent/refractory MPNST remain elusive. To address this gap, we conducted a retrospective analysis of our institutional experience in treating patients with relapsed MPNST in order to describe patient outcomes related to salvage regimens.
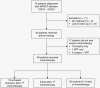
Methods: We conducted a retrospective electronic health record analysis of patients with MPNST who were treated at Johns Hopkins Hospital from January 2010 to June 2021. We calculated time to progression (TTP) based on salvage chemotherapy regimens.
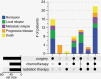
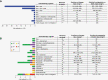
Results: Sixty-five patients were included in the analysis. Upfront therapy included single or combined modalities of surgery, chemotherapy, or radiotherapy. Forty-eight patients received at least 1 line of chemotherapy, which included 23 different regimens (excluding active clinical studies). Most patients (n = 42, 87.5%) received a combination of doxorubicin, ifosfamide, or etoposide as first-line chemotherapy. Salvage chemotherapy regimens and their TTP varied greatly, with irinotecan/temozolomide-based regimens having the longest average TTP (255.5 days, among 4 patients).
Conclusions: Patients with advanced or metastatic MPNST often succumb to their disease despite multiple lines of therapy. These data may be used as comparative information in decision-making for future patients and clinical trials.
Keywords: chemotherapy; malignant peripheral nerve sheath tumors; neurofibromatosis type 1; salvage therapy; soft tissue sarcoma.
© The Author(s) 2023. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Conflict of interest statement
K.M.L is an inventor on the patent (WO2022046910) for prodrugs of 6-Mercaptopurine (not relevant to the current manuscript). C.A.P. is an inventor on the patent application (WO2022234409A1) held/submitted by the Johns Hopkins University and Novartis that covers compounds and compositions for the treatment of MPNST (not relevant to the current manuscript). C.A.P. is a recipient of research grants from Novartis (not relevant to the current manuscript) and Kura Oncology (not relevant to the current manuscript) and has received consulting fees from Day One Therapeutics and Genentech (not relevant to the current manuscript). C.F.M. received royalties from UpToDate (not relevant to the current manuscript). J.O.B is a national co-investigator for clinical trials supported by Alexion, SpringWorks, and Takeda (none are relevant to this current manuscript). The other co-authors declare that they have no competing interests.
Figures

Similar articles
-
Systemic Options for Malignant Peripheral Nerve Sheath Tumors.Curr Treat Options Oncol. 2021 Feb 27;22(4):33. doi: 10.1007/s11864-021-00830-7. Curr Treat Options Oncol. 2021. PMID: 33641042 Review.
-
Systemic Treatment for Advanced and Metastatic Malignant Peripheral Nerve Sheath Tumors-A Sarcoma Reference Center Experience.J Clin Med. 2020 Sep 29;9(10):3157. doi: 10.3390/jcm9103157. J Clin Med. 2020. PMID: 33003503 Free PMC article.
-
Management and prognosis of malignant peripheral nerve sheath tumors: The experience of the French Sarcoma Group (GSF-GETO).Eur J Cancer. 2016 Mar;56:77-84. doi: 10.1016/j.ejca.2015.12.015. Epub 2016 Jan 26. Eur J Cancer. 2016. PMID: 26824706
-
Early outcomes for malignant peripheral nerve sheath tumor treated with chemotherapy.Am J Clin Oncol. 2011 Aug;34(4):417-21. doi: 10.1097/COC.0b013e3181e9c08a. Am J Clin Oncol. 2011. PMID: 20838322
-
Malignant Peripheral Nerve Sheath Tumors of the Brachial Plexus: A Single-Center Experience on Diagnosis, Management, and Outcomes.Ann Plast Surg. 2023 Apr 1;90(4):339-342. doi: 10.1097/SAP.0000000000003462. Epub 2023 Feb 4. Ann Plast Surg. 2023. PMID: 36752552 Review.
References
-
- Bates JE, Peterson CR, Dhakal S, Giampoli EJ, Constine LS.. Malignant peripheral nerve sheath tumors (MPNST): a SEER analysis of incidence across the age spectrum and therapeutic interventions in the pediatric population. Pediatr Blood Cancer. 2014;61(11):1955–1960. - PubMed
-
- Lewis JJ, Brennan MF.. Soft tissue sarcomas. Curr Probl Surg. 1996;33(10):817–872. - PubMed
-
- Carli M, Ferrari A, Mattke A, et al. . Pediatric malignant peripheral nerve sheath tumor: the Italian and German soft tissue sarcoma cooperative group. J Clin Oncol. 2005;23(33):8422–8430. - PubMed
-
- Anghileri M, Miceli R, Fiore M, et al. . Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer. 2006;107(5):1065–1074. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
