Patient-derived glioblastoma organoids reflect tumor heterogeneity and treatment sensitivity
- PMID: 38130902
- PMCID: PMC10733660
- DOI: 10.1093/noajnl/vdad152
Patient-derived glioblastoma organoids reflect tumor heterogeneity and treatment sensitivity
Abstract
Background: Treatment resistance and tumor relapse are the primary causes of mortality in glioblastoma (GBM), with intratumoral heterogeneity playing a significant role. Patient-derived cancer organoids have emerged as a promising model capable of recapitulating tumor heterogeneity. Our objective was to develop patient-derived GBM organoids (PGO) to investigate treatment response and resistance.
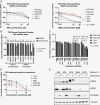
Methods: GBM samples were used to generate PGOs and analyzed using whole-exome sequencing (WES) and single-cell karyotype sequencing. PGOs were subjected to temozolomide (TMZ) to assess viability. Bulk RNA sequencing was performed before and after TMZ.
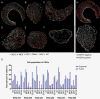
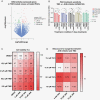
Results: WES analysis on individual PGOs cultured for 3 time points (1-3 months) showed a high inter-organoid correlation and retention of genetic variants (range 92.3%-97.7%). Most variants were retained in the PGO compared to the tumor (range 58%-90%) and exhibited similar copy number variations. Single-cell karyotype sequencing demonstrated preservation of genetic heterogeneity. Single-cell multiplex immunofluorescence showed maintenance of cellular states. TMZ treatment of PGOs showed a differential response, which largely corresponded with MGMT promoter methylation. Differentially expressed genes before and after TMZ revealed an upregulation of the JNK kinase pathway. Notably, the combination treatment of a JNK kinase inhibitor and TMZ demonstrated a synergistic effect.
Conclusions: Overall, these findings demonstrate the robustness of PGOs in retaining the genetic and phenotypic heterogeneity in culture and the application of measuring clinically relevant drug responses. These data show that PGOs have the potential to be further developed into avatars for personalized adaptive treatment selection and actionable drug target discovery and as a platform to study GBM biology.
Keywords: glioblastoma; organoids; preclinical models; temozolomide resistance; tumor heterogeneity.
© The Author(s) 2023. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Conflict of interest statement
None declared.
Figures



References
-
- Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
-
- Hegi ME, Diserens AC, Gorlia T, et al. . MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
