Differentially expressed genes in orbital adipose/connective tissue of thyroid-associated orbitopathy
- PMID: 38130930
- PMCID: PMC10734407
- DOI: 10.7717/peerj.16569
Differentially expressed genes in orbital adipose/connective tissue of thyroid-associated orbitopathy
Abstract
Background: Thyroid-associated orbitopathy (TAO) is a disease associated with autoimmune thyroid disorders and it can lead to proptosis, diplopia, and vision-threatening compressive optic neuropathy. To comprehensively understand the molecular mechanisms underlying orbital adipogenesis in TAO, we characterize the intrinsic molecular properties of orbital adipose/connective tissue from patients with TAO and control individuals.
Methods: RNA sequencing analysis (RNA-seq) was performed to measure the gene expression of orbital adipose/connective tissues of TAO patients. Differentially expressed genes (DEGs) were detected and analyzed through Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis, and Gene Set Enrichment Analysis (GSEA). The protein-protein interaction (PPI) network was constructed using the STRING database, and hub genes were identified by the Cytoscape plug-in, cytoHubba. We validated several top DEGs through quantitative real-time polymerase chain reaction (qRT-PCR).
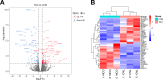
Results: We identified 183 DEGs in adipose tissue between TAO patients (n = 3) and control patients (n = 3) through RNA sequencing, including 114 upregulated genes and 69 downregulated genes. The PPI network of these DEGs had 202 nodes and 743 edges. PCR-based validation results of orbital adipose tissue showed multiple top-ranked genes in TAO patients (n = 4) are immune and inflammatory response genes compared with the control individual (n = 4). They include ceruloplasmin isoform x3 (CP), alkaline tissue-nonspecific isozyme isoform x1 (ALPL), and angiotensinogen (AGT), which were overrepresented by 2.27- to 6.40-fold. Meanwhile, protein mab-21-like 1 (MAB21L1), phosphoinositide 3-kinase gamma-subunit (PIK3C2G), and clavesin-2 (CLVS2) decreased by 2.6% to 32.8%. R-spondin 1 (RSPO1), which is related to oogonia differentiation and developmental angiogenesis, was significantly downregulated in the orbital muscle tissues of patients with TAO compared with the control groups (P = 0.024).
Conclusions: Our results suggest that there are genetic differences in orbital adipose-connective tissues derived from TAO patients. The upregulation of the inflammatory response in orbital fat of TAO may be consistent with the clinical phenotype like eyelid edema, exophthalmos, and excess tearing. Downregulation of MAB21L1, PIK3C2G, and CLVS2 in TAO tissue demonstrates dysregulation of differentiation, oxidative stress, and developmental pathways.
Keywords: Differentially expressed genes; High-throughput sequencing; Inflammation; Thyroid Associated Ophthalmopathy; mRNA.
©2023 Wang et al.
Conflict of interest statement
The authors declare there are no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

