Improved transition metal photosensitizers to drive advances in photocatalysis
- PMID: 38131090
- PMCID: PMC10732135
- DOI: 10.1039/d3sc04580c
Improved transition metal photosensitizers to drive advances in photocatalysis
Abstract
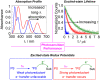
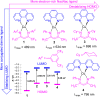
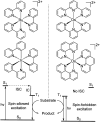
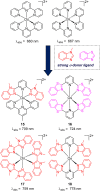
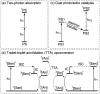
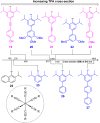
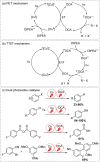
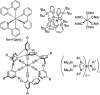
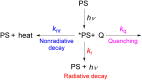
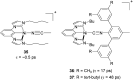
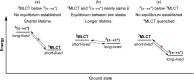
To function effectively in a photocatalytic application, a photosensitizer's light absorption, excited-state lifetime, and redox potentials, both in the ground state and excited state, are critically important. The absorption profile is particularly relevant to applications involving solar harvesting, whereas the redox potentials and excited-state lifetimes determine the thermodynamics, kinetics, and quantum yields of photoinduced redox processes. This perspective article focuses on synthetic inorganic and organometallic approaches to optimize these three characteristics of transition-metal based photosensitizers. We include our own work in these areas, which has focused extensively on exceptionally strong cyclometalated iridium photoreductants that enable challenging reductive photoredox transformations on organic substrates, and more recent work which has led to improved solar harvesting in charge-transfer copper(i) chromophores, an emerging class of earth-abundant compounds particularly relevant to solar-energy applications. We also extensively highlight many other complementary strategies for optimizing these parameters and highlight representative examples from the recent literature. It remains a significant challenge to simultaneously optimize all three of these parameters at once, since improvements in one often come at the detriment of the others. These inherent trade-offs and approaches to obviate or circumvent them are discussed throughout.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






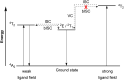
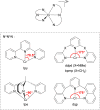
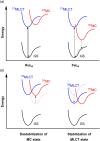
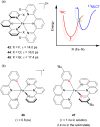
References
-
- V. Gold, The IUPAC Compendium of Chemical Terminology: The Gold Book, International Union of Pure and Applied Chemistry (IUPAC), Research Triangle Park, NC, 4th edn, 2019
-
- Wu Y. Kim D. Teets T. S. Synlett. 2021:1390–9065.
-
- Juris A. Balzani V. Barigelletti F. Campagna S. Belser P. von Zelewsky A. Coord. Chem. Rev. 1988;84:85–277. doi: 10.1016/0010-8545(88)80032-8. - DOI
-
- Teets T. S. and Wu Y., in Comprehensive Organometallic Chemistry IV, Elsevier, 2022. 284–338
Publication types
LinkOut - more resources
Full Text Sources

