Microglia facilitate and stabilize the response to general anesthesia via modulating the neuronal network in a brain region-specific manner
- PMID: 38131301
- PMCID: PMC10746144
- DOI: 10.7554/eLife.92252
Microglia facilitate and stabilize the response to general anesthesia via modulating the neuronal network in a brain region-specific manner
Abstract
General anesthesia leads to a loss of consciousness and an unrousable state in patients. Although general anesthetics are widely used in clinical practice, their underlying mechanisms remain elusive. The potential involvement of nonneuronal cells is unknown. Microglia are important immune cells in the central nervous system (CNS) that play critical roles in CNS function and dysfunction. We unintentionally observed delayed anesthesia induction and early anesthesia emergence in microglia-depleted mice. We found that microglial depletion differentially regulates neuronal activities by suppressing the neuronal network of anesthesia-activated brain regions and activating emergence-activated brain regions. Thus, microglia facilitate and stabilize the anesthesia status. This influence is not mediated by dendritic spine plasticity. Instead, it relies on the activation of microglial P2Y12 and subsequent calcium influx, which facilitates the general anesthesia response. Together, we elucidate the regulatory role of microglia in general anesthesia, extending our knowledge of how nonneuronal cells modulate neuronal activities.
Keywords: P2Y12; anesthesia; calcium; microglia; mouse; neuroscience.
© 2023, He, Liu, He et al.
Conflict of interest statement
YH, TL, QH, WK, XL, JD, SD, ZS, JW, BY, YW, YM, YR, YS, BP No competing interests declared
Figures
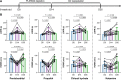




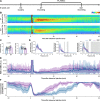


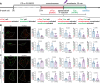

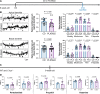
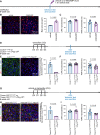

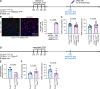

Update of
- doi: 10.1101/2023.10.06.561235
- doi: 10.7554/eLife.92252.1
Comment in
- doi: 10.7554/elife.95064
References
-
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, Roth BL. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. - DOI - PMC - PubMed
-
- Alves M, Gomez-Villafuertes R, Delanty N, Farrell MA, O’Brien DF, Miras-Portugal MT, Hernandez MD, Henshall DC, Engel T. Expression and function of the metabotropic purinergic P2Y receptor family in experimental seizure models and patients with drug-refractory epilepsy. Epilepsia. 2017;58:1603–1614. doi: 10.1111/epi.13850. - DOI - PubMed
-
- Badimon A, Strasburger HJ, Ayata P, Chen X, Nair A, Ikegami A, Hwang P, Chan AT, Graves SM, Uweru JO, Ledderose C, Kutlu MG, Wheeler MA, Kahan A, Ishikawa M, Wang Y-C, Loh Y-HE, Jiang JX, Surmeier DJ, Robson SC, Junger WG, Sebra R, Calipari ES, Kenny PJ, Eyo UB, Colonna M, Quintana FJ, Wake H, Gradinaru V, Schaefer A. Negative feedback control of neuronal activity by microglia. Nature. 2020;586:417–423. doi: 10.1038/s41586-020-2777-8. - DOI - PMC - PubMed
-
- Bedolla A, Mckinsey G, Ware K, Santander N, Arnold T, Luo Y. Finding the Right Tool: A Comprehensive Evaluation of Microglial Inducible Cre Mouse Models. bioRxiv. 2023 doi: 10.1101/2023.04.17.536878. - DOI
MeSH terms
Grants and funding
- STI2030-Major Projects 2022ZD0204700/Ministry of Science and Technology of the People's Republic of China
- STI2030-Major Projects 2022ZD0207200/Ministry of Science and Technology of the People's Republic of China
- STI2030-Major Projects 2021ZD0202500/Ministry of Science and Technology of the People's Republic of China
- 32170958/National Natural Science Foundation of China
- 32000678/National Natural Science Foundation of China
- 32130044/National Natural Science Foundation of China
- T2241002/National Natural Science Foundation of China
- 32100930/National Natural Science Foundation of China
- 32200953/National Natural Science Foundation of China
- Program of Shanghai Academic/Technology Research Leader 21XD1420400/Shanghai Municipal People's Government
- 22SG07/Shanghai Shuguang Program
- Shanghai Pilot Program for Basic Research 21TQ014/Shanghai Municipal People's Government
- The Innovative Research Team of High-Level Local University in Shanghai/Shanghai Municipal People's Government
- Shanghai Municipal Science and Technology Major Project (2018SHZDZX01)/Shanghai Municipal People's Government
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

