Z-Form Adoption of Nucleic Acid is a Multi-Step Process Which Proceeds through a Melted Intermediate
- PMID: 38131335
- PMCID: PMC11155437
- DOI: 10.1021/jacs.3c10406
Z-Form Adoption of Nucleic Acid is a Multi-Step Process Which Proceeds through a Melted Intermediate
Abstract
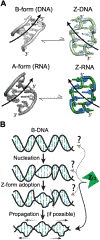
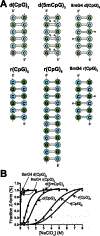
The left-handed Z-conformation of nucleic acids can be adopted by both DNA and RNA when bound by Zα domains found within a variety of innate immune response proteins. Zα domains stabilize this higher-energy conformation by making specific interactions with the unique geometry of Z-DNA/Z-RNA. However, the mechanism by which a right-handed helix contorts to become left-handed in the presence of proteins, including the intermediate steps involved, is poorly understood. Through a combination of nuclear magnetic resonance (NMR) and other biophysical measurements, we have determined that in the absence of Zα, under low salt conditions at room temperature, d(CpG) and r(CpG) constructs show no observable evidence of transient Z-conformations greater than 0.5% on either the intermediate or slow NMR time scales. At higher temperatures, we observed a transient unfolded intermediate. The ease of melting a nucleic acid duplex correlates with Z-form adoption rates in the presence of Zα. The largest contributing factor to the activation energies of Z-form adoption as calculated by Arrhenius plots is the ease of flipping the sugar pucker, as required for Z-DNA and Z-RNA. Together, these data validate the previously proposed "zipper model" for Z-form adoption in the presence of Zα. Overall, Z-conformations are more likely to be adopted by double-stranded DNA and RNA regions flanked by less stable regions and by RNAs experiencing torsional/mechanical stress.
Figures





References
-
- Wolfram S Principles of Nucleic Acid Structure, 1st ed.; Springer; New York: New York, 1984.
Figure references
-
- Schwartz T; Rould MA; Lowenhaupt K; Herbert A; Rich A Crystal Structure of the Zalpha Domain of the Human Editing Enzyme ADAR1 Bound to Left-Handed Z-DNA. Science (80-. ). 1999, 11 (284(5421)), 1841–1845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

